Chromatography
Chromatography is a key technique in chemistry used to separate and study the components of a mixture. It works by moving different substances at different speeds through a medium, allowing scientists to identify and measure the amounts of each component. This method is important in many fields, including chemistry, biology, and environmental science. Chromatography also helps scientists understand how substances interact with each other and identify unknown compounds. [1-4]
Principles of Chromatography
Chromatography involves two main parts: the mobile phase and the stationary phase. The mobile phase is a fluid that moves through the system, carrying the sample with it. The stationary phase stays in one place and doesn’t move. The key idea behind chromatography is that different components in a mixture interact differently with these two phases. [1,2]
When the sample is introduced, its components either stick to the stationary phase or move with the mobile phase. How much each component sticks or moves depends on its specific properties, like size, charge, or how well it dissolves. Components that stick more to the stationary phase move slower, while those that move more with the mobile phase travel faster. This difference in movement causes the components to separate from each other.
By choosing the right mobile and stationary phases and adjusting conditions like temperature or pressure, scientists can achieve precise separation of the components. After they are separated, these components can be collected, studied, or identified.
Types of Chromatography [1,2]
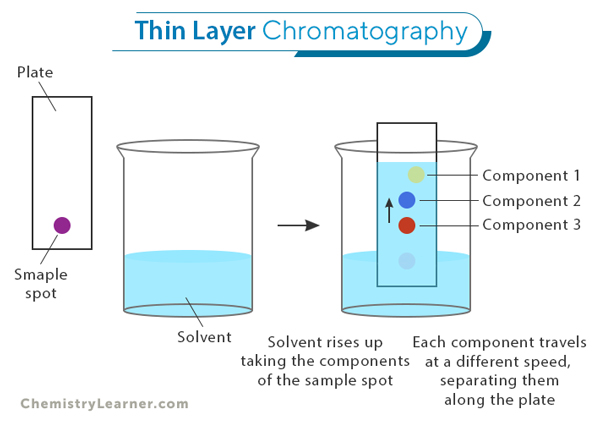
Thin Layer Chromatography (TLC): TLC is a simple and affordable technique where a thin layer of material, like silica gel, is spread on a glass or plastic plate. It is commonly used to identify compounds, check the progress of chemical reactions, and quickly separate mixtures. While TLC provides fast results, it might not separate components as precisely as other methods.
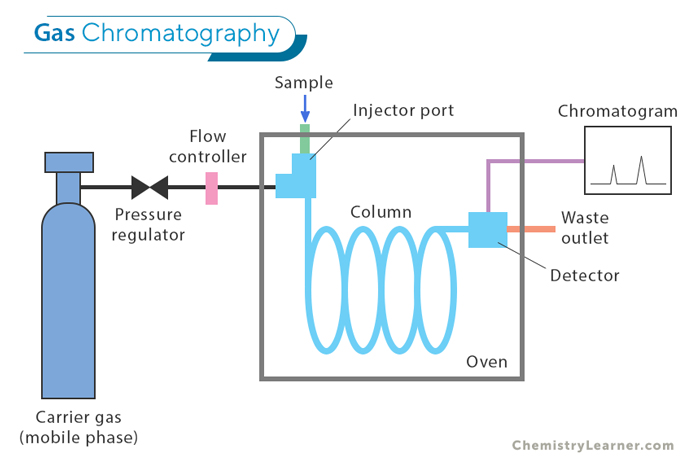
Gas Chromatography (GC): This technique is used to separate and analyze compounds that easily turn into gas, called volatile compounds. In GC, the sample is heated until it becomes a vapor and then injected into a column filled with a material that doesn’t move (stationary phase). A gas (the mobile phase) pushes the vapor through the column. GC is very sensitive and can separate compounds with great detail, making it useful for analyzing chemicals in industries like environmental testing, forensics, and medicine.
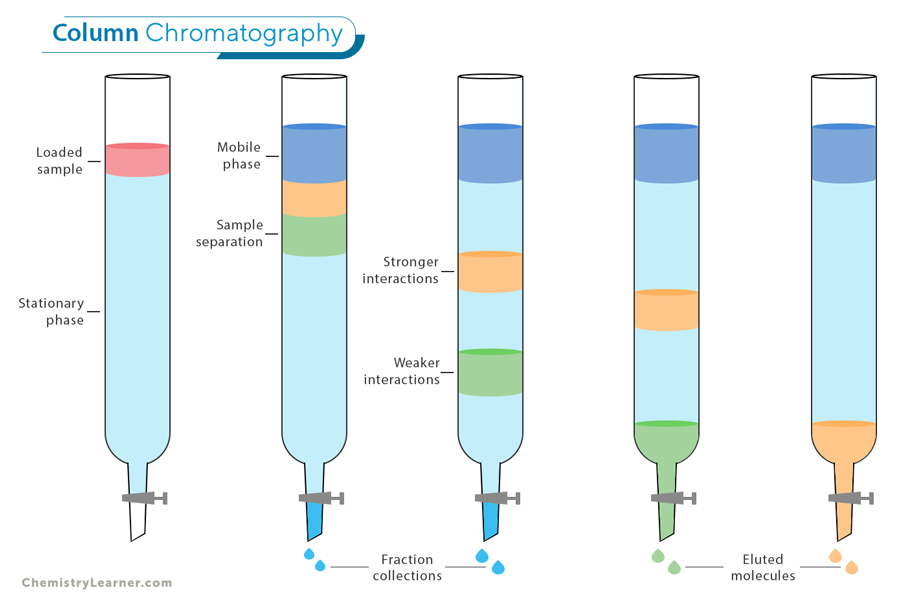
Column Chromatography: This technique separates the components of a mixture by passing it through a column packed with a solid material, known as the stationary phase. As the mixture moves down the column, different components travel at different speeds based on their interactions with the stationary phase, allowing them to be separated. Column chromatography is widely used in laboratories for purifying compounds, isolating specific substances, and analyzing complex mixtures in fields such as chemistry, biology, and pharmaceuticals.
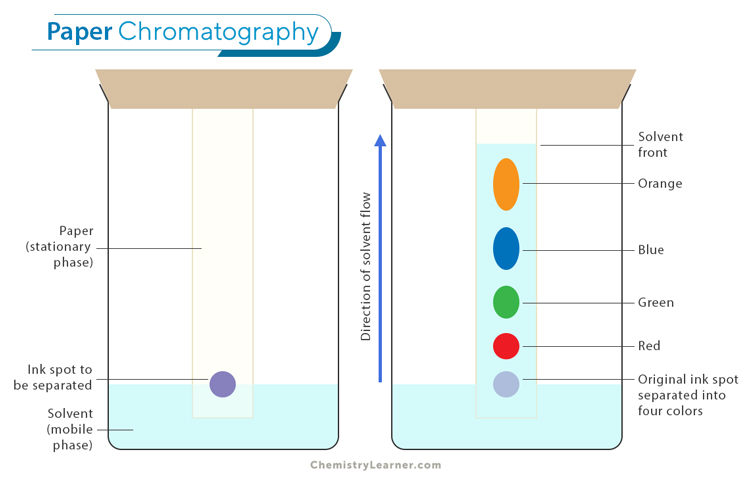
Paper Chromatography: Paper chromatography is a simple and cost-effective technique used to separate mixtures, especially for identifying pigments and other compounds in a sample. In this method, a drop of the mixture is placed on a piece of paper, and a solvent is allowed to move through the paper by capillary action. As the solvent travels, it carries the different components of the mixture at different rates, causing them to separate into distinct spots along the paper. This method is often used in educational settings and for preliminary analysis in laboratories.
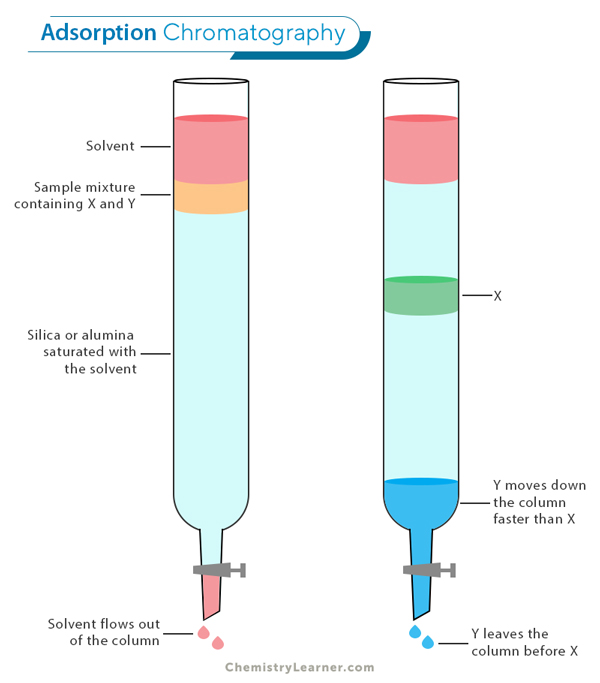
Adsorption Chromatography: In this type of chromatography, the separation of components is based on how strongly they adsorb, or stick, to the surface of a solid stationary phase. As the mobile phase (a liquid or gas) carries the sample mixture through the system, different components interact with the stationary phase to varying degrees. Components that adsorb more strongly move slower through the system, while those with weaker adsorption move faster, resulting in separation. Adsorption chromatography is commonly used in various industries to purify compounds and analyze mixtures.
Partition Chromatography: This technique separates components of a mixture based on their different affinities for a liquid stationary phase and a liquid mobile phase. In partition chromatography, the sample is introduced into a column containing a liquid stationary phase coated on a solid support. As the sample moves through the column with the mobile phase, components interact with the stationary phase to varying degrees, causing them to separate. This method is particularly useful for analyzing and purifying compounds that are soluble in the liquid phases and is widely applied in chemical analysis and research. Paper chromatography is a type of partition chromatography
Applications [1]
- Purification: Chromatography is used in laboratories to purify compounds, obtaining pure samples from mixtures.
- Quantification: It helps quantify reactants or products and assess their percentage purity.
- Experimental Use: Analytical chemists use chromatography for various experiments, including detecting and isolating pure compounds.
- Food and Water Testing: It monitors the presence of pesticides, fungicides, and contaminants in food and drinking water and examines contaminant levels in manufactured food.
- Forensic Science: Chromatography plays a crucial role in forensic science investigations.
FAQs
Ans. Elution is extracting compounds or molecules from a stationary phase by passing a solvent or a mobile phase through it. The eluent is the solvent or mobile phase that moves through the stationary phase, carrying the target compounds. The eluate is the resulting solution that contains the separated or purified compounds after they have been eluted from the stationary phase by the eluent.