Redox Reaction (Oxidation-Reduction Reaction)
What is a Redox Reaction?
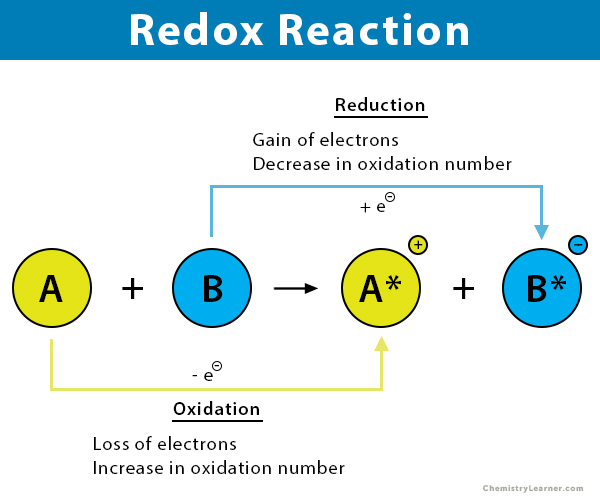
A redox reaction is a chemical reaction in which the atoms change their oxidation numbers. Some atoms lose electrons and are oxidized – a process known as oxidation. On the other hand, some atoms gain electrons and are reduced – a process known as reduction. Therefore, both REDuction and OXidation take place simultaneously, hence the term redox. Redox reactions usually occur in one of two environments: acidic or basic [1-3].
Identifying Redox Reactions
A way to recognize a redox reaction is to look for a change in the oxidation number of the two elements involved in the reaction. The oxidized element increases its oxidation number, while the reduced element decreases its oxidation number. By checking this change, one can quickly determine and tell whether the reaction is redox or not.
What Happens in a Redox Reaction?
In a redox reaction, electrons move from one atom to another, which changes the oxidation number or state of the two atoms. The oxidation state of an element corresponds to the number of electrons an atom loses, gains, or appears to use when combining with other atoms in compounds.
Redox Half-Reaction
A half-reaction is part of an overall reaction that separately represents either an oxidation or a reduction. Two half-reactions, including one oxidation and one reduction, must describe a redox reaction completely [4].
Example
When a nickel (Ni) strip is placed in an aqueous solution of copper (II) sulfate (CuSO4), an immediate reaction occurs. Copper metal begins to deposit on the strip. The only source of metallic copper in this system is the copper (II) ions (Cu2+) in the solution. The reaction is represented as follows.
Cu2+ (aq.) + 2e– → Cu(0) (s)
Here, the Cu2+ ion gains two electrons and is reduced to Cu (0). So, the above equation represents the reduction part of a redox reaction, and hence, it is called a redox half-reaction.
On the other hand, nickel releases two electrons to form nickel (II) ions (Ni2+). These are the two electrons that go to copper.
Ni(0} (s) → Ni2+ (aq.) + 2e–
Thus, nickel is oxidized, and the above half-reaction represents the oxidation part of the redox reaction.
Balancing Redox Reaction using Half-Reaction [5,6]
The half-reaction can be used to balance the redox reaction. Consider an example of the combustion redox reaction between magnesium (Mg) and oxygen (O2), resulting in magnesium oxide (MgO). The unbalanced equation is given by:
Mg (s) + O2 (g) → MgO (s)
Mg loses two electrons and becomes an Mg2+ cation. Its oxidation number goes from zero to +2. Hence, it is oxidized. The oxidation part of the reaction is written as:
Mg (s) → Mg2+ + 2 e–
On the other hand, O gains two electrons from Mg and becomes O2-, thereby changing its oxidation number from zero to -2. Hence, oxygen is reduced, and the reduction part of the reaction is:
½ O2 (g) + 2 e– → O2-
Adding the two equations together:
Mg (s) + ½ O2 (g) → MgO (s)
Multiplying both sides by 2:
2 Mg (s) + O2 (g) → 2 MgO (s)
This balanced equation represents the reaction between magnesium and oxygen.
Examples of Redox Reaction
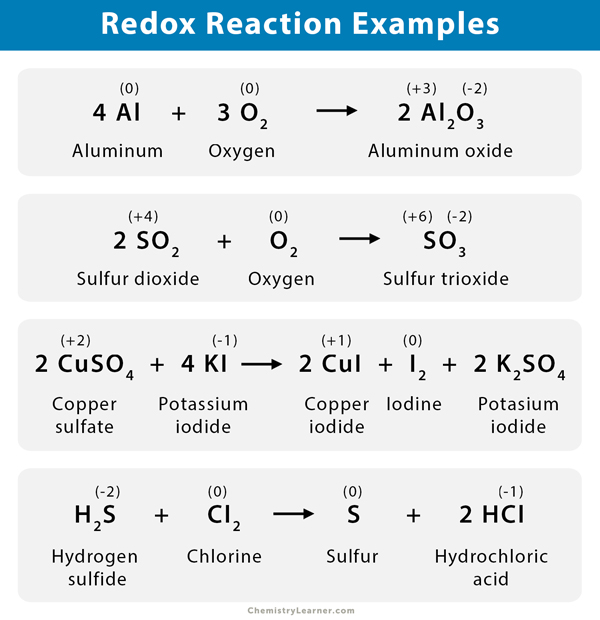
Below are some examples of the redox reaction with explanations [1-9].
1. The reaction between lead oxide (PbO) and ammonia (NH3) results in nitrogen (N2), water (H2O), and lead (Pb).
3 PbO (s) + 2 NH3 (g) → N2 (g) + 3 H2O (l) + 3 Pb (s)
The oxidation number of lead decreases from +2 to 0, and Pb is reduced. The oxidation number of nitrogen increases from -3 to zero, meaning N is oxidized.
2. The reaction between carbon (C) and Iron (III) oxide (Fe2O3) results in iron (Fe) and carbon dioxide (CO2).
3 C (s) + 2 Fe2O3 (s) → 4 Fe (s) + 3 CO2 (g)
The oxidation number of carbon goes from zero to +4, and C is oxidized. The oxidation number of iron decreases from +3 to zero, and Fe is reduced.
3. In a thermite reaction, iron (Fe) atoms in ferric oxide (Fe2O3) lose oxygen (O) atoms to aluminum (Al) atoms, producing aluminum oxide (Al2O3).
Fe2O3 (s) + 2 Al (s) → Al2O3 (s) + 2 Fe (l)
The oxidation number of aluminum goes from zero to +3 (oxidation), and that of iron decreases from +3 to zero (reduction).
4. Chromium (Cr) reacts with hydrochloric acid (HCl) to give chromium chloride (CrCl2) and hydrogen (H2) gas.
Cr (s) + 2 HCl (aq.) → CrCl2 (aq.) + H2 (g)
The oxidation number of chromium increases from zero to +2 (oxidation), and that of hydrogen decreases from +1 to zero (reduction).
5. Sulfur dioxide (SO2) reacts with carbon monoxide (CO) to form sulfur (S) and carbon dioxide (CO2).
SO2 (g) + 2 CO (g) → S (g) + 2 CO2 (s)
The oxidation number of sulfur decreases from +4 to zero (reduction), and that of carbon increases from +2 to +4 (oxidation).
6. In the reaction between manganese (IV) oxide (MnO2) and hydrochloric acid (HCl), the products are manganese (II) chloride (MnCl2), chlorine (Cl2) gas, and water (H2O).
MnO2 (s) + 4 HCl (aq.) → MnCl2 (aq.) + Cl2 (g) + 2 H2O (l)
The oxidation number of manganese decreases from +4 to +2 (reduction), and that of chlorine increases from -1 to zero (oxidation).
Types of Redox Reactions
The following list represents the four types of redox reactions.
- Combination reaction – two reactants combine to form a product
- Decomposition reaction – a compound decomposes into two or more products
- Single-replacement reaction – one atom replaces another atom to form two products
- Disproportionation reaction – a decomposition reaction in which the same atom is simultaneously oxidized and removed
Common Uses of Redox Reaction in Everyday Life
Redox reactions have many applications in industry and daily life, with many benefits and effects [10].
- Conversion of food and oxygen to carbon dioxide and water (cellular respiration, including glycolysis)
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + Energy
- Functioning of batteries in all electronic devices
- Photosynthesis – reduction of carbon dioxide into sugars and the oxidation of water into molecular oxygen
6 CO2 + 6 H2O + light energy → C6H12O6 + 6 O2
- Burning of organic material and combustion of hydrocarbons in fossil fuels
- Extraction of ores from minerals
- Electroplating
- Developing a photographic film
- Bleaching and decolorization
- Corrosion
- Breathalyzer
FAQs
Ans. If a positive voltage is formed across the electrodes in a voltaic cell, the redox reaction is a spontaneous reaction for reduction at the cathode and oxidation at the anode.
Ans. Redox reactions are important because they are the principal sources of energy for a living cell. Oxidation of molecules by removal of hydrogen or combination with oxygen liberates typically large quantities of energy.
Ans. It is an acidic medium if H+ is present while balancing the reaction. If OH– is present, then it is a basic medium.
Ans. Balancing redox reactions is different from balancing other reactions. A half-reaction is used in a redox reaction where the number of atoms and the amount of charge must be balanced.
Ans. Yes, corrosion is a redox reaction.