Chemical Reactions
What is a Chemical Reaction?
A chemical reaction is a process in which one or more substances are converted to one or more different substances. The starting substances are called the reactants, and the new substances that form are called the products.
What Happens During a Chemical Reaction?
A chemical reaction can include atoms, ions, compounds, or molecules of a single element. During a chemical reaction, chemical bonds between the atoms break in the reactants, forming new chemical bonds in the products. The atoms rearrange to form new bonds. As the chemical bonds break, the positions of electrons change, resulting in products with properties that are different from the properties of the reactants.
Characteristics of a Chemical Reaction
There are ways to identify a chemical reaction. The signs that indicate a reaction are called indicators of a chemical reaction. The breaking and formation of bonds are considered essential characteristics for the occurrence of a chemical reaction. Therefore, the characteristics of a chemical reaction include:
- change in color
- formation of a precipitate
- formation of a gas
- odor change
- temperature change
How to Write a Chemical Reaction?
A chemical reaction is written in an equation using the chemical symbol of the element or compound participating in the reaction process. The reactants are written on the left, and the products are written on the right. An arrow separates the two. The coefficient in front of a compound represents the number of moles that are being consumed or formed. The subscript represents the number of atoms of a particular element present in the compound. Finally, balancing the equation ensures that the relationship between the reactants and the product is correct.
How to Balance a Chemical Reaction?
Step 1: Identify each element found in the equation. The number of atoms of the element must be the same on each side of the equation.
Step 2: Check the net charge on each side of the equation. The net charge must be the same on each side.
Step 3: Start with an element found in one compound on each side of the equation and find its number of atoms. Change the coefficient so the number of atoms is the same on each side of the equation. Do not change the subscript.
Step 4: Repeat the process with another element after balancing one element. Proceed until all elements have been balanced. It is easiest to leave elements found in pure form for last.
Step 5: Check the equation once more and ensure the charge and the number of elements on both sides of the equation are balanced.
Example
Hydrogen (H2) and oxygen (O2) combine to produce water (H2O).
H2 + O2 → H2O
This reaction is unbalanced. To balance it, first multiply the oxygen by ½, as shown below:
H2 + ½ x O2 → H2O
Next, multiply both sides by two so that the coefficients are whole numbers and the equation is balanced:
2 H2 + O2 → 2 H2O
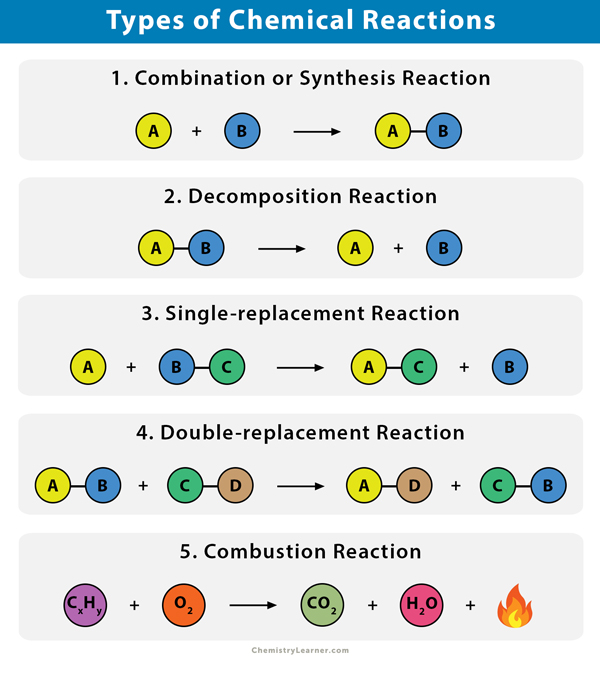
Different Types of Chemical Reactions
Many chemical reactions can be classified into one of five basic types. A thorough understanding of these types of reactions is useful for predicting the products of an unknown reaction. The five basic types of chemical reactions are combination, decomposition, single-replacement, double-replacement, and combustion [1 – 5].
1. Synthesis (Combination) Reaction
In a synthesis or combination reaction, two or more substances combine to form a single new substance. Combination reactions are called synthesis reactions.
A + B → AB
Examples
Na (s) + Cl2 (g) → NaCl (s)
- Magnesium (Mg) rapidly reacts when ignited with oxygen (O2) to produce a fine powder of magnesium oxide (MgO).
2 Mg (s) + O2 (g) → 2 MgO (s)
2. Decomposition Reaction
A compound breaks down into two or more simple substances in a decomposition reaction.
AB → A + B
Examples
CaCO3 (s) → CaO (s) + CO2 (g)
- Sodium hydroxide (NaOH) decomposes to produce sodium oxide (Na2O) and water (H2O).
2 NaOH (s) → Na2O (s) + H2O (g)
3. Single-replacement or Single-displacement Reaction
In a single-replacement reaction, one element replaces a similar element in a compound.
A + BC → AC + B
Examples
- Zinc (Zn) reacts with hydrochloric acid (HCl) to produce aqueous zinc chloride (ZnCl2) and hydrogen (H2).
Zn (s) + 2 HCl (aq.) → ZnCl2 (aq.) + H2 (g)
- When a strip of magnesium (Mg) metal is placed in an aqueous solution of copper (II) nitrate (CuNO3), it replaces copper, resulting in aqueous magnesium nitrate (MgNO3) and solid copper (Cu) metal.
Mg (s) + Cu(NO3)2 (aq.) → Mg(NO3)2 (aq.) + Cu (s)
4. Double-replacement or Double-displacement Reaction
In a double-replacement reaction, the positive and negative ions of two ionic compounds exchange places to form two new compounds.
AB + CD → AD + CB
The double-replacement reaction is of two types.
The formation of an insoluble solid in an aqueous solution is called a precipitation reaction. The solid is called a precipitate.
Examples
- When aqueous solutions of potassium iodide (KI) and lead (II) nitrate (Pb(NO3)2) are mixed, insoluble lead iodide (PbI2) forms in aqueous potassium nitrate (K2NO3).
2 KI (aq.) + Pb(NO3)2 (aq.) → K2NO3 (aq.) + PbI2 (s/ppt.)
- A solution of potassium chloride (KCl) and silver nitrate (AgNO3) forms a white insoluble solid, silver chloride (AgCl), in the resulting solution of potassium nitrate (KNO3).
2 KCl (aq.) + AgNO3 (aq.) → KNO3 (aq.) + AgCl (s/ppt.)
b) Acid-Base Reaction or Neutralization Reaction
The reaction between an acid and a base is called an acid-base or neutralization reaction and forms water.
Example
- A mixture of sulfuric acid (H2SO4) and sodium hydroxide (NaOH) produces sodium sulfate (Na2SO4) and water (H2O).
H2SO4 (aq.) + 2 NaOH (aq.) → Na2SO4 (aq.) + 2 H2O (l)
5. Combustion Reaction
A combustion reaction occurs when a substance reacts with oxygen gas (O2), releasing energy through light and heat. Oxygen must be present for a combustion reaction to take place.
Examples
- The combustion of hydrogen (H2) gas in the presence of oxygen (O2) produces water vapor (H2O).
2 H2 (g) + O2 (g) → 2 H2O (g)
- The burning of coal (carbon) in oxygen (O2) gives carbon dioxide (CO2).
C (s) + O2 (g) → CO2 (g)
- Propane (C3H8), a gaseous hydrocarbon, is commonly used as the fuel source in gas grills. It combusts in oxygen (O2) to give carbon dioxide (CO2) and water (H2O).
C3H8 (g) + 5 O2 (g) → 3 CO2 (g) + 4 H2O (g)
Note that the above examples are common chemical reactions in everyday life.
Other Types of Chemical Reactions
1. Redox Reaction
The redox reaction is an oxidation-reduction reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing electrons. The atom that loses electrons is said to have oxidized, and the atom that gains electrons is reduced. Redox reactions can be synthesis, decomposition, single-replacement, or combustion reactions. However, not all combustion reactions are redox reactions.
Example
- Iron (Fe) reacts with oxygen (O2) to give iron (III) oxide. Thus, the oxidation number of iron goes from zero to +3.
4 Fe + 3 O2 → 2 Fe2O3
The above reaction is also a combination reaction. Oxygen is called the oxidizing agent since it oxidizes iron to iron (III) oxide.
2. Polymerization
Polymerization is a process in which relatively small molecules, called monomers, combine chemically to produce a vast chainlike or network molecule called a polymer. This process is known as a chain reaction.
Example
- The polymerization of ethylene (C2H4) produces polyethylene.
C2H4 + C2H4 + C2H4 + — → (C2H4)n
3. Hydrolysis
Hydrolysis is the process of adding water to break down a molecule into two parts.
Example
- Dissolving sulfuric acid (H2SO4) in water (H2O) yields bisulfate (HSO3–) and hydronium (H3O+)
H2SO4 + H2O → HSO3– + H3O+
4. Dehydration Synthesis
Dehydration synthesis is the opposite of hydrolysis. Here, two molecules combine to form a new molecule and eliminate water.
Example
- Dehydration of ethanol (C2H5OH) at 170 ⁰C gives ethene (C2H4)
CH3 – CH2 – OH → CH2 = CH2 + H2O
5. Photochemical Reaction
A photochemical reaction is a type of chemical reaction in which the reactants take in energy in the form of photons from a source of light, like the sun, to form products.
Example
- Photography uses the action of light on grains of silver chloride (AgCl) to produce an image. Silver chloride (AgCl) decomposes into silver (Ag) and chlorine (Cl2) gas.
2 AgCl + hν → 2 Ag + Cl2
6. Endothermic Reaction
A chemical reaction is said to be endothermic when it absorbs heat from the surroundings.
Example
- Limestone or calcium carbonate (CaCO3) decomposes when heated to a high temperature. Quick lime or calcium oxide (CaO) and carbon dioxide (CO2) are the products.
CaCO3 (s) + heat → CaO (s) + CO2 (g)
7. Exothermic Reaction
A chemical reaction is said to be exothermic when it releases heat to the surroundings.
Example
- Calcium oxide (CaO) reacts vigorously with water (H2O) to produce calcium hydroxide (Ca(OH)2) or slacked lime.
CaO (s) + H2O (l) → Ca(OH)2 (aq.) + heat
Rate of a Chemical Reaction
In a chemical reaction, the reactants are consumed to give products – the concentration of the reactants decreases, and the concentration of the products increases. The speed or rate of a chemical reaction is the change in concentration of the reactant or product per unit time.
Factors Affecting the Rate of Chemical Reactions
These are some of the factors affecting the rate of chemical reactions.
- Reactant concentration
- The physical state of the reactants and surface area
- Temperature
- Presence of a catalyst – A catalyst is a substance that can activate and speed up a chemical reaction.
Some chemical reactions are also reversible, i.e., the products recombine to give back the reactants. In this case, both the forward and the reverse reactions will have their rates.
Chemical Reactions in Everyday Life
Considering the abundance of substances in and around us, observing examples of chemical reactions in everyday life is not unusual. Some of the prominent examples are:
- Respiration – Human beings inhale oxygen and release carbon dioxide. It is an example of an exothermic reaction.
- Photosynthesis – Plants use sunlight to convert carbon dioxide to oxygen, a type of photochemical reaction.
- Rusting – Iron gradually reacts with oxygen and oxidizes to form iron (III) oxide, commonly known as rust.
- Burning – Combustion of fuel takes place exothermically in vehicles and factories.
FAQs
Ans. Fire is a result of combustion that produces various gases like carbon dioxide, sulfur dioxide, nitrogen dioxide, water vapor, smoke, and other gaseous substances, depending on the source of the fire. Hence, it is a chemical reaction.
Ans. Matter consists of atoms. During a chemical reaction, atoms are never created or destroyed. The mass of the reactants is equal to that of the products. Hence, mass or matter is conserved during a chemical reaction.
Ans. In any chemical reaction, chemical bonds in the reactants are broken, and new bonds in the products are formed. Therefore, in order to effectively initiate a reaction, the reactants must be moving fast enough, with enough energy, so that they collide with sufficient force for bonds to break.
Ans. No. Only physical changes take place during the melting of ice. There is no chemical change.
Ans. Yes. Burning a candle is a combustion reaction.
Ans. No. The boiling of water is a physical change and not a chemical change.
Ans. Nuclear reactions involve changing an atom’s nucleus, producing a different element. Chemical reactions, on the other hand, involve only a rearrangement of electrons and do not involve changes in the nuclei.
Ans. An exploding firework consists of several chemical reactions that are happening simultaneously or in rapid sequence. Two simple reactions that occur in fireworks are combustion and oxidation. Combustion supplies the heat required for quick oxidation.