Double Covalent Bond
What is a Double Covalent Bond?
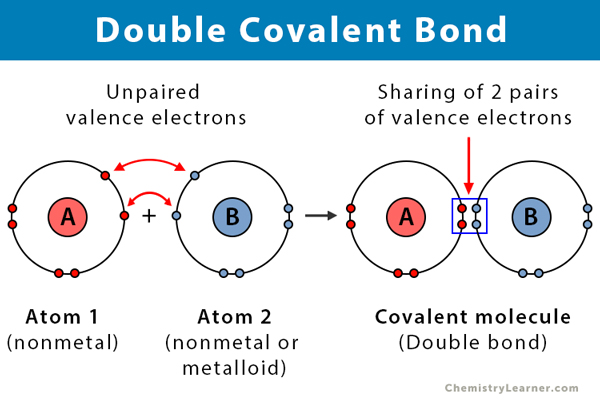
A covalent bond is a type of chemical bond in which the atoms in a compound share electrons. A double covalent bond, or merely a double bond, involves the sharing of two pairs of electrons, i.e., four electrons. Atoms combine in order to complete their valence (outermost) shell and become stable. They bond due to strong electrostatic attraction between the bonding electrons and the nuclei of both the atoms. This type of bond is prevalent in alkenes [1-3].
Properties of Double Covalent Bond
- Takes place between two nonmetals or a nonmetal and a metalloid
- Consists of two pairs of electrons, i.e., four electrons
- Intermediate bond length
- Unstable
Examples of Double Covalent Bond
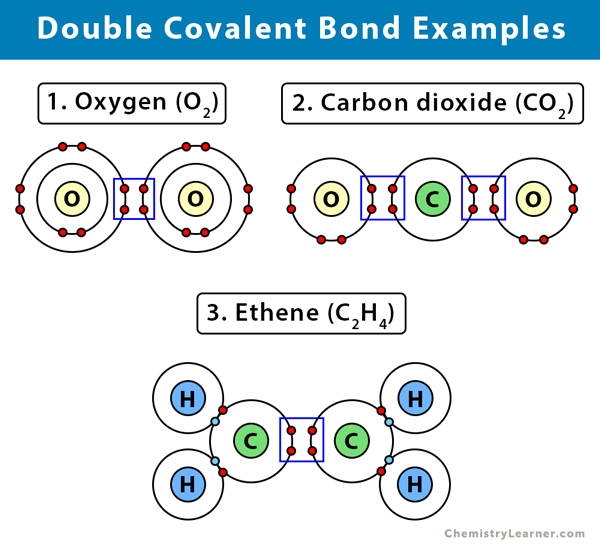
Here are some examples of a double covalent bond [1-4].
1. Oxygen (O2)
A molecule with a double covalent bond is oxygen. The oxygen molecule consists of two oxygen (O) atoms. Each oxygen has only six electrons and requires two more to complete its outermost shell. Therefore, the two electrons from each oxygen bond together. By sharing the four electrons, the oxygen molecule displays a double covalent bond.
2. Carbon dioxide (CO2)
Carbon (C) has only four electrons on its outermost shell. It requires four more electrons to complete the shell. Oxygen (O) has only six electrons and requires two more. Therefore, two oxygen atoms will share two of their six valence electrons with carbon. The result is two double covalent bonds between carbon and oxygen.
3. Ethene (C2H4)
An example of an alkene is ethane. Ethane has two carbon (C) and four hydrogen (H) atoms. Carbon (C) has four valence electrons in its outermost shell. It requires four more to fill up its orbital. Hydrogen (H) has a lone electron and requires one more to complete its orbital. Two hydrogen atoms will combine with one carbon atom to form two single bonds. Two other hydrogen atoms will combine with another carbon, resulting in two single bonds. However, the two carbon atoms still have two unpaired electrons each. They will combine, and each will share the two electrons, making it a total of four shared electrons. As a result, a double bond will form.