Polarity of Dichloromethane (CH2Cl2)
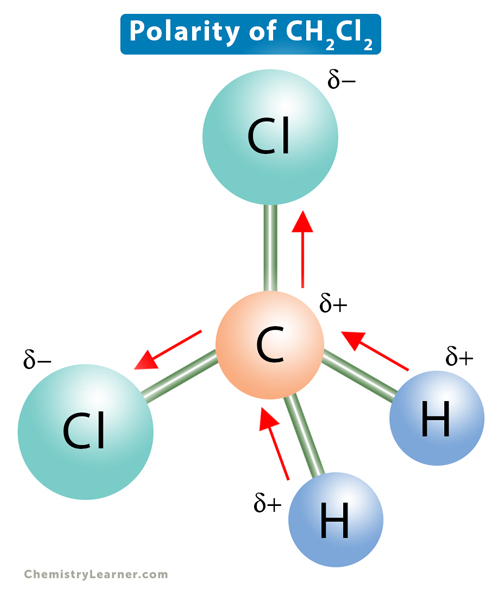
Dichloromethane (CH2Cl4) consists of a central carbon (C) atom with a coordination number 4. Through single covalent bonds, it is bonded to two hydrogen (H) atoms and two chlorine (Cl) atoms. The bonds coordinate around carbon so that the distance between them is maximum. At the same time, the repulsion between the bonding pairs of electrons is minimum. The such arrangement leads to a tetrahedral geometry with a bond angle of 109.5° [1-4].
The electronegativity of carbon is 2.55, that of chlorine is 3.16, and that of hydrogen is 2.2. The electronegativity difference between C and Cl is 0.61, and between C and H is 0.35. Therefore, the C-Cl bonds are more polar than the C-H bonds, so there is some net residual polarity. The bond dipoles are arranged asymmetrically. There is no way to arrange the molecule such that the dipole moments cancel out. As a result, there will be a net dipole moment, making dichloromethane a polar molecule.