Polarity of Hydrogen Sulfide (H2S)
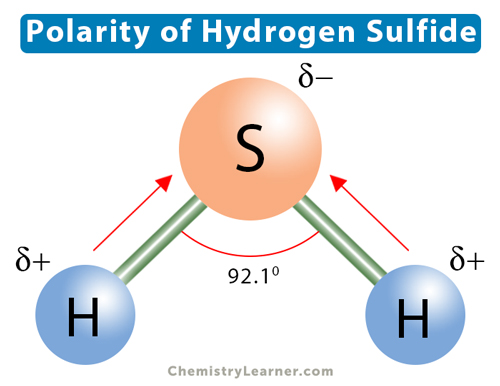
Hydrogen sulfide (H2S) is a covalent compound made from two hydrogen (H) atoms bonded to a central sulfur (S) atom. Two single covalent bonds are formed in the molecule. The hybridization in H2S is sp3 with tetrahedral geometry. Sulfur has two pairs of nonbonding electrons. The lone pairs repel the bonded electrons, pushing the S-H bonds inward. As a result, the bond angle is 92.1° instead of the usual 109.28° for tetrahedral. The decrease in angle leads to a bent or v-shaped structure, as shown below [1-4].
The electronegativity of hydrogen is 2.2, and that of sulfur is 2.58. Therefore, the electronegativity difference is 0.38. It makes the S-H bond slightly polar. The asymmetric bent shape also makes the H2S molecule slightly polar.