Polarity of Boron Trifluoride (BF3)
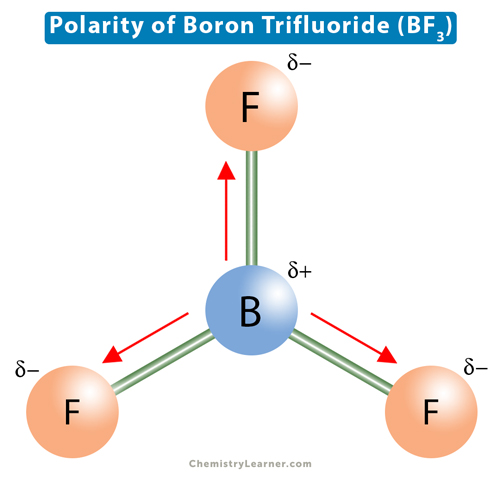
Boron trifluoride (BF3) is composed of a boron (B) atom bonded by three fluoride (F) atoms through single covalent bonds. The molecular geometry of BF3 is a trigonal planer with three peripheral atoms surrounding one central atom in a single plane. The bond lengths of the three B-F bonds are similar, and all the bonds are separated by 120°. The molecular structure of BF3 is such that the bonds combine to form an equilateral triangle, making BF3 a highly symmetrical molecule [1-4].
Each B-F bond is polar due to the electronegativity difference between B and F, meaning the electrons are not shared equally by the boron and the fluorine atoms. Instead, they are pulled toward the electronegative fluorine atom. A partial negative charge appears on fluorine, and a partial positive charge appears on boron, resulting in a dipole moment. However, due to the symmetrical shape of the BF3 molecule, all three B-F bond dipole moments cancel. The net dipole moment is zero, making boron trifluoride nonpolar.