Heat of Sublimation
The heat of sublimation is the enthalpy change when one gram of a solid directly converts into vapor at a constant temperature. If one mole of the substance transforms, we call the enthalpy change molar heat of sublimation [1-4].
Molecular Explanation of Sublimation
A typical household substance is naphthalene balls. When naphthalene balls are in a solid phase, their molecules do not have intermolecular space. The molecules do not have any kinetic energy. It means there is no motion of particles. However, the molecules randomly move when they gain energy, increasing their intermolecular space and changing the solid balls to the gaseous phase [2].
How to Calculate Heat of Sublimation
A solid transforms into vapor during sublimation without going through the liquid phase. Energy must be supplied to the substance to sublimate. The energy added goes into doing the following [1]:
- Excite the substance so that it achieves the desired heat capacity at a solid state
- Break all the intermolecular bonds holding the solid
- Excited the free molecules so that the solid substance transforms into gas with minimum heat capacity
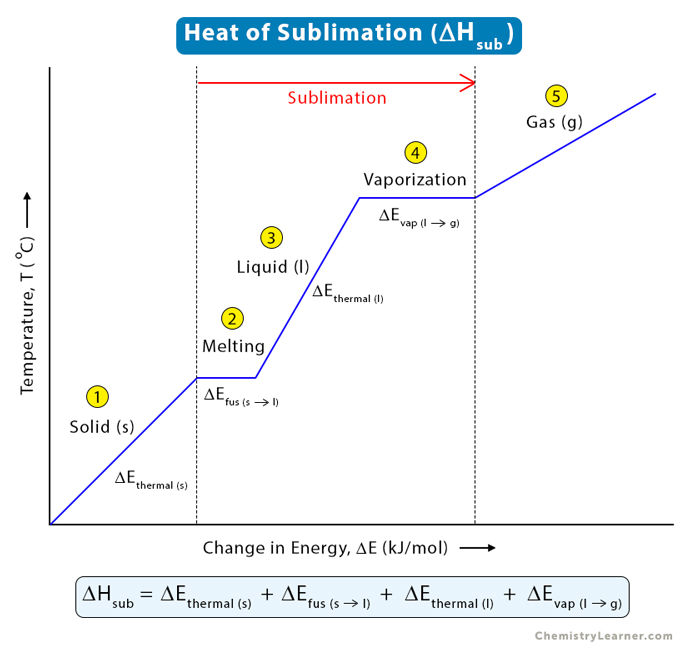
To calculate the heat of sublimation, we construct a temperature vs. energy change graph for the sublimation process. In this graph, we show the fusion and the vaporization stages. Since enthalpy is a state function, we add all the energies associated with the solid, liquid, and gas phases. Let us look at what energies are involved throughout the entire process.
Thermal Energy
When a substance is heated, it absorbs heat. The amount of heat absorbed is given by the change in thermal energy.
ΔEThermal = m cp ΔT
Where
ΔEThermal: Change in thermal energy
m: Mass
CP: Specific heat
ΔT: Change in temperature
Bond Energy
Bond energy is the energy required to separate the molecules so that the substance can undergo a phase change from a lower energy state to a higher energy state. By phase change, we mean melting and vaporization.
ΔEBond = (Δm) (ΔHphase change)
Where
ΔEBond: Bond energy
Δm: Change in mass
ΔHphase change: Enthalpy change due to phase change
Therefore, the various energy changes involved in the sublimation process are:
- ΔEThermal (s): Thermal energy in the solid phase
- ΔEfus (s →l): Enthalpy of fusion
- ΔEThermal (l): Thermal energy in the liquid phase
- ΔEvap (l →v): Enthalpy of vaporization
The sum of all the energy changes gives the heat of sublimation (ΔHsub).
ΔHsub = ΔEThermal (s) + ΔEfus (s →l) + ΔEThermal (l) + ΔEvap (l →v)
Unit: J/g or J/mol
Example Problem
Calculate the heat required to convert 1.00 kg of ice with the initial temperature of 273 K into steam at 373 K. Given: heat of fusion = 333.55 kJ/kg, specific heat of water = 4.18 J/g·K, and heat of vaporization = 2257 kJ/kg. Also, calculate the heat of sublimation of 1 mole of ice whose molar mass is 18.02 g.
Solution
Let us break down the sublimation process into various steps.
Step 1: Conversion of 1.00 kg ice into water at 273 K
ΔEfus (s → l) = 333.55 kJ/kg x 1.00 kg = 333.55 kJ
Step 2: Heating of 1.00 kg water from 273 K to 373 K
ΔEthermal (l) = m cP ΔT = 1000 g x 4.18 J/g·K x (373 K – 273 K) = 418 kJ
Step 3: Conversion of 1.00 kg water into steam at 373 K
ΔEvap (l → g) = 2257 kJ/kg x 1.00 kg = 2257 kJ
The heat necessary is
ΔHsub = ΔEfus (s → l) + ΔEthermal (l) + ΔEvap (l → g) = 333.55 kJ + 418 kJ + 2257 kJ = 3008 kJ
The molar heat of sublimation of ice is
ΔHsub = 3008 kJ/kg x 18.02 x 10-3 kg/mol = 54.2 kJ/mol