Dipole Moment
The dipole moment is the measure of the polarity of a molecule. It occurs when there is a separation of charges. A dipole moment exists in a single atom or molecule. When it occurs in a molecule, it can be between two atoms in an ionic or covalent bond [1-4].
If the two atoms have a significant electronegativity difference, the dipole moment is permanent, and the molecule is polar. The dipole moment is low if the two atoms have a slight electronegativity difference. However, it can be increased by bringing a charged species near it. The resulting moment is known as an induced dipole moment.
Dipole moment is a valuable concept in dielectrics and has applications in solid and liquid materials.
Equation
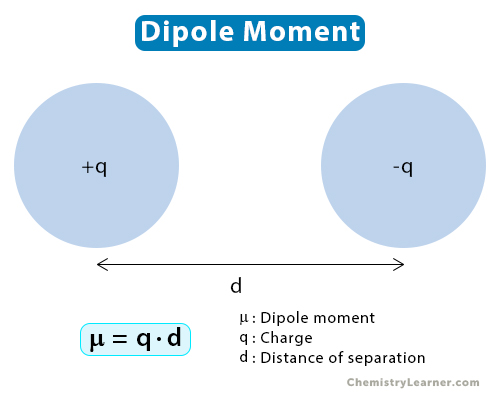
Consider two point charges of magnitude q separated by a distance d. The mathematical expression for the dipole moment (μ) is [1-6]
μ = q·d
The Greek symbol μ (mu) represents the dipole moment. From the above equation, it is pretty clear that the magnitude of the charges and their distance of separation are the deciding factors.
Unit
Since the product of change and distance gives the dipole moment, its SI unit is Coulomb · meter (C·m). However, a more convenient unit is Debye or D, which is given by
1 D = 3.33564 × 10-30 C·m
1 D is the typical dipole moment of a molecule.
Polyatomic Molecules
A molecule consists of several atoms. Each pair of atoms will have a bond dipole moment due to chemical bonding, represented by magnitude and direction. However, the molecule’s overall dipole moment will depend upon the magnitude and direction of the individual bond dipole moments. Therefore, the net moment is the vector addition of the individual moments.
μ = Σqiri
Where
μ: dipole moment of the molecule
qi: charge of the i-th atom
ri: distance of the i-th atom from a reference point
Aside, there are other factors that the total molecular dipole moment depends upon. These are
- The difference in sizes of the two atoms
- Hybridization of the orbitals
- Presence of lone pair
There are two critical considerations for a molecule to have a dipole. First, it must have polar bonds. Second, its shape should be such that the individual bond dipoles do not cancel out. Based on these facts, let us look at the dipole moments of a few molecules.
Examples [1-6]
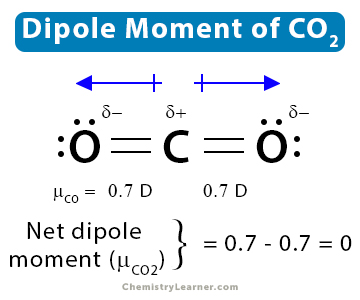
1. Carbon dioxide (CO2)
CO2 has a linear structure, O=C=O, and displays symmetry. There are dipole moments due to the C-O bonds. However, as the structure is linear, the dipole moment of one bond is canceled by the other. Therefore, the net dipole moment of CO2 is zero.
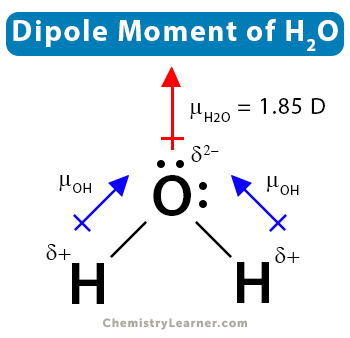
2. Water (H2O)
Water consists of one oxygen (O) and two hydrogen (H) atoms. Oxygen shares one electron with each hydrogen (H) atom. Water has a bent structure with an H-O-H bond angle of 104.5˚. This is why the individual dipole moments do not cancel out. Therefore, an asymmetrical molecule like water has a nonzero dipole moment. Its value is 1.85 D.
Dipole Moments Charts
The following table gives the dipole moment of some well-known bonds.
| Bond | Dipole Moment (D) |
|---|---|
| H-C | 0.4 |
| H-N | 1.3 |
| H-O | 1.5 |
| H-F | 1.7 |
| H-Cl | 1.1 |
| H-Br | 0.8 |
| H-I | 0.4 |
| C-C | 0 |
| C-N | 0.2 |
| C-O | 0.7 |
| C-F | 1.6 |
| C-Cl | 1.5 |
| C-Br | 1.4 |
| C-I | 1.2 |
The following table gives the dipole molecule of some well-known molecules [7].
| Molecule Type | Example | Dipole Moment (D) | Geometry |
|---|---|---|---|
| HF | 1.78 | Linear | |
| HCl | 1.07 | Linear | |
| AB | HBr | 0.79 | Linear |
| HI | 0.38 | Linear | |
| H2 | 0 | Linear | |
| H2O | 1.85 | Bent | |
| AB2 | H2S | 0.95 | Bent |
| CO2 | 0 | Linear | |
| NH3 | 1.47 | Pyramidal | |
| AB3 | NF3 | 0.23 | Trigonal pyramid |
| BF3 | 0 | Trigonal planar | |
| CH4 | 0 | Tetrahedral | |
| AB4 | CHCl3 | 1.04 | Tetrahedral |
| CCl4 | 0 | Tetrahedral |