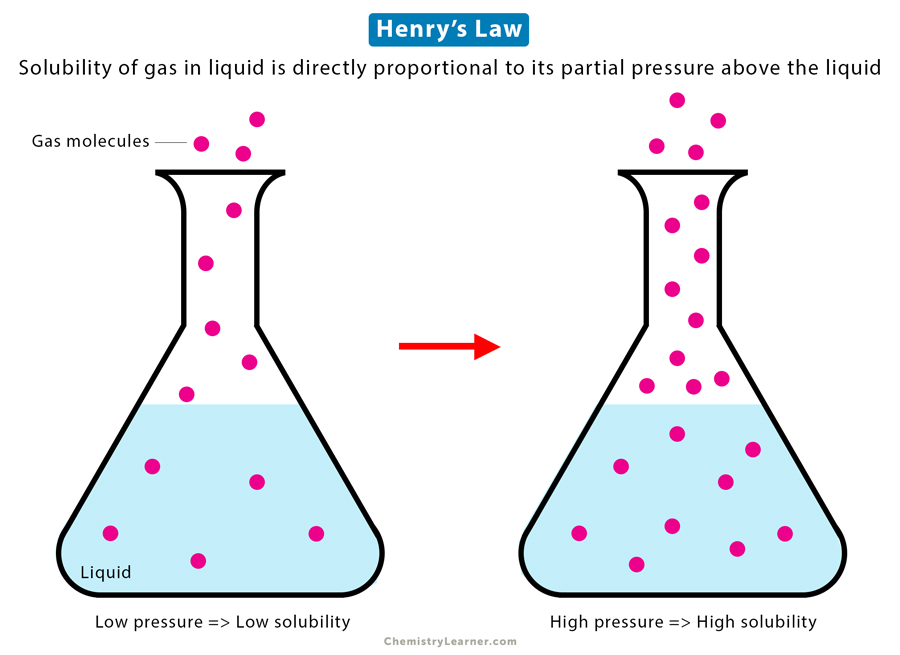
Henry’s Law
Henry’s Law states that the amount of gas dissolved in a given type and volume of liquid is directly proportional to its partial pressure above the liquid. In other words, the solubility of the gas at a constant temperature is proportional to its partial pressure [1-4].
According to Henry’s Law, if the gas pressure over the liquid increases, the amount of gas dissolved in the liquid will increase proportionally. Conversely, as the gas pressure decreases, the amount of dissolved gas drops.
This law is named after English chemist William Henry, who published his findings in 1803.
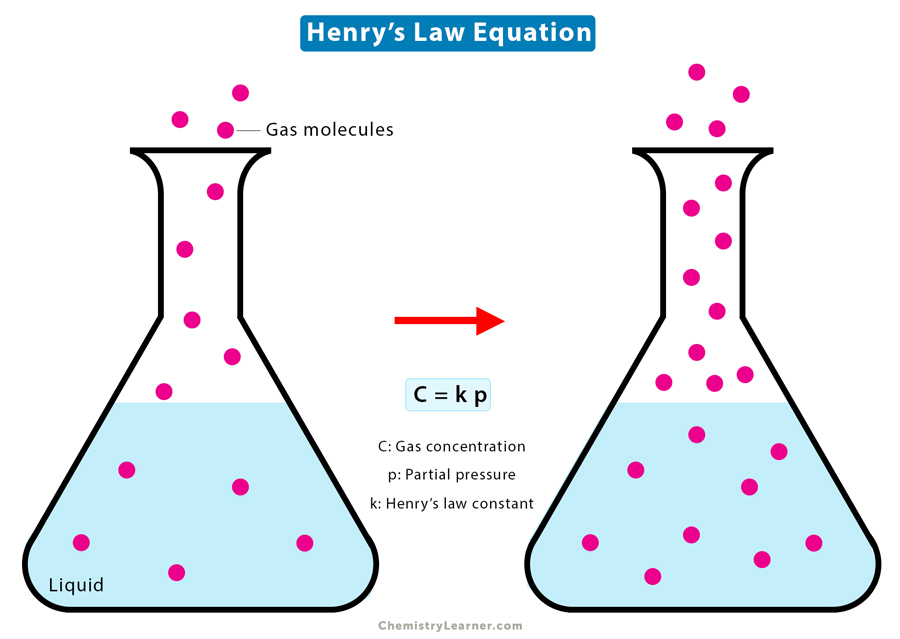
Henry’s Law Equation
Henry’s Law is based on the assumption that the gas behaves like an ideal gas. Mathematically, Henry’s Law is written as follows [1-4].
C = k Pg
Where
C: Solubility of the gas at a constant temperature
Pg: Partial pressure of the gas
k: Proportionality constant, also known as Henry’s Law constant
Unit of Henry’s Law constant: mol·m-3·Pa-1 or mol·L-1·atm-1
Examples of Henry’s Law [4]
1. Carbonated Drinks
An everyday example of Henry’s law is carbonated soft drinks. Carbonated beverages consist of highly compressed carbon dioxide (CO2) gas. CO2 pressure above the liquid is above the standard atmospheric pressure, resulting in high solubility.
When the bottle or can is opened, the pressurized gas escapes into the atmosphere, accompanied by a “fizz” and a hissing sound. The CO2 pressure above the liquid decreases along with the solubility. As a result, the dissolved CO2 rises inside the liquid as air bubbles and escapes into the atmosphere. Suppose the drink is kept open for too long. In that case, the CO2 concentration inside comes to equilibrium with the CO2 concentration in the atmosphere (~0.05%). The “fizzing” effect stops, and the drink loses its taste.
2. Respiration
During inhalation, oxygen (O2) partial pressure increases in the alveoli. When the deoxygenated blood reaches the oxygen-rich atmosphere in the alveoli, the following exchanges take place.
- Since the O2 partial pressure in the alveoli is high and the oxygen concentration in the deoxygenated blood is low, O2 flows from the former to the latter.
- The CO2 partial pressure in the alveoli is relatively low and that of the standard atmospheric pressure (~0.05%). Since the dissolved CO2 in the deoxygenated blood is high, it moves to the alveoli. The gas is then expelled from the lungs through exhalation.
List of Henry’s Law Constant
The table below shows a list of Henry’s Law constants for a few prevalent gasses in water [2].
| Gas in Water | Henry’s Law Constant (mol·m-3·Pa-1) |
|---|---|
| Hydrogen bromide (HBr) | 0.24 |
| Perchloric acid (HClO4) | 9.9 x 103 |
| Hydrogen Fluoride (HF) | 1.3 x 102 |
| Nitric Acid (HNO3) | 2.1 x 103 |
| Hydrogen (H2) | 7.7 x 10-6 |
| Deuterium (D2) | 7.9 x 10-6 |
| Oxygen (O2) | 1.2 x 10-5 |
| Bromine (Br2) | 7.2 x 10-3 |
| Sulfur Dioxide (SO2) | 1.2 x 10-2 |
| Methane (CH4) | 1.4 x 10-5 |
| Benzene (C6H6) | 1.8 x 10-3 |
Applications of Henry’s Law [4]
- It can be used where multiple gasses are present. Environmental researchers commonly utilize Henry’s Law to examine gasses passing back and forth between the atmosphere and Earth’s water systems.
- It is used to determine the amount of dissolved oxygen and nitrogen in the blood of divers and assess the risk of decompression sickness.
Limitations of Henry’s Law [2]
- The law is applicable when the gas is in equilibrium with the liquid. If the gas-liquid system is not in equilibrium, then the gas concentration in the solution will be inaccurate.
- The law applies when the gas does not react with the liquid. Henry’s Law will fail if there is a chemical reaction between the two.
- The law breaks down at high concentration and pressure.
FAQs
Ans. Boyle’s law refers to the relationship between the pressure exerted by a gas and its volume. On the other hand, Henry’s law refers to a relationship between a gas’s concentration and its partial pressure.