Third Law of Thermodynamics
The third law of thermodynamics concerns the behavior of atoms and molecules at absolute zero temperature. It states that the entropy of a system is zero as the temperature approaches absolute zero. In other words, the entropy of a perfect crystal of a pure substance is zero at zero Kelvin (0 K) [1-4].
German chemist Walter Nernst discovered the third law of thermodynamics between the years 1906 to 1912. Hence, the third law is sometimes called the Nernst theorem or Nernst postulate.
Significance
The significance of the third law is that it allows us to measure the absolute entropy of any pure substance at any temperature, using zero Kelvin as the reference temperature. However, it is impossible to achieve a temperature of zero Kelvin. No matter how cold a system is, it can discretely be made colder but can never reach absolute zero [2].
According to Nernst, “it is impossible for a process to bring a given system’s entropy to zero in a discrete and finite number of steps.”
Third Law and Perfect Crystal
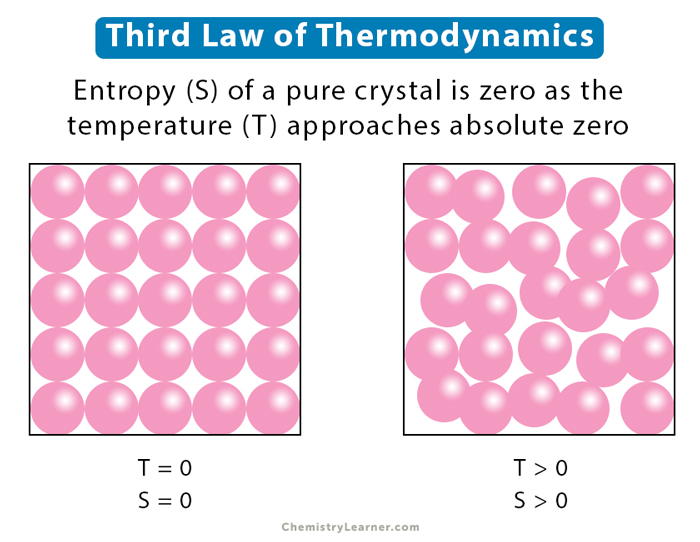
A perfect crystal is one in which every molecule is undistinguishable, and the molecular alignment is flawless throughout the substance. For impure crystals, or those with faulty alignment, some energy will be associated with the imperfections, so the entropy cannot become zero [1-3].
Equation
According to the third law of thermodynamics, the entropy change for a physical or chemical transformation approaches zero as the temperature approaches absolute zero. Mathematically, it is written as follows [2,4,5]:
Lim ΔS = 0 as T → 0
At zero Kelvin, the system does not contain any heat. All atoms and molecules are at their lowest energy points. It means the system has only one accessible microstate, the ground state. According to Boltzmann equation, the entropy as a function of the number of microstates corresponding to each macrostate (W) is
S = K ln W
A perfect crystal has only one unique ground state. Therefore, W = 1 and S = K ln 1 = 0. Thus, a perfect crystal’s entropy at 0 K is zero.
Example
We can take the example of water to demonstrate the third law of thermodynamics. Water in its gaseous state has molecules that can move around very freely – it has high entropy. As the gas cools, it becomes liquid. The molecules can still move around, but not as freely as gas. During the condensation process, they lose some entropy [1,3,5].
Water cools further, resulting in solid ice. The ice molecules can no longer move freely but can only vibrate within the lattice. The entropy is now very low. As the water is cooled further, bringing the temperature closer to absolute zero, the vibration of the molecules diminishes. If the solid water reaches absolute zero, all molecular motion ceases completely. At this point, water does not have any entropy at all.