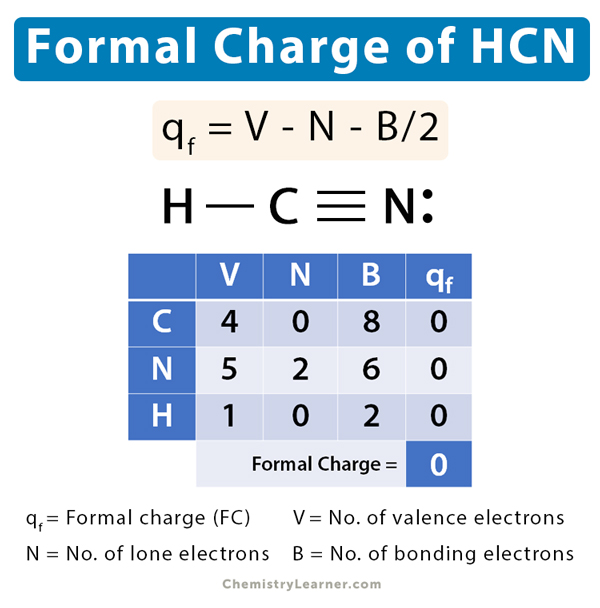
Hydrogen Cyanide (HCN) Formal Charge
In the hydrogen cyanide (HCN) molecule, the central carbon (C) atom shares a single covalent bond with the hydrogen (H) atom and a triple covalent bond with nitrogen (N). Its stable Lewis structure is shown below.
Let us calculate the formal charge of HCN by determining the formal charges on C, H, and N.
V = 4, N = 0, B = 8
Therefore, formal charge on carbon is given by,
qf = 4 – 0 – 8/2 = 0
The formal charge on C in HCN is zero.
V = 5, N = 2, B = 6
Therefore, formal charge on nitrogen is given by,
qf = 5 – 2 – 6/2 = 0
The formal charge on N in HCN is zero.
V = 1, N = 0, B = 2
Therefore, formal charge on hydrogen is given by,
qf = 1 – 0 – 1/2 = 0
The net formal charge is: 0 + 0 + 0 = 0
The formal charge of hydrogen cyanide is zero.