Born-Haber Cycle
Born-Haber cycle is a process that allows one to understand and analyze energies in a chemical reaction. It is concerned with forming a stable ionic compound from reacting an alkali or alkaline earth metal with a nonmetal like a halogen or oxygen. The process enables one to understand the overall reaction progress through steps by visually representing the energies involved in these steps [1 – 4].
The Born-Haber cycle is named after two German scientists, Max Born and Fritz Haber, who developed it in 1919.
Born-Haber Cycle and Lattice Energy
Born-Haber cycle is used primarily to calculate lattice energy, which cannot typically be measured directly. Lattice energy is the energy released when one mole of an ionic compound is formed from its constituents in their gaseous state. It is due to the electrostatic force of attraction and is responsible for keeping the anions and cations of the compound in fixed positions [1 – 4].
Born-Haber cycle uses Hess’s Law to find the lattice energy exclusively. The formation of a solid ionic compound from the elemental state of the constituent atoms goes through several steps and involves enthalpy in each step. Hess’ law states that the enthalpy change in a chemical process is independent of the path the process takes. When summed over the individual steps, the total enthalpy change over a closed cycle is zero.
How to Use Born Haber Cycle
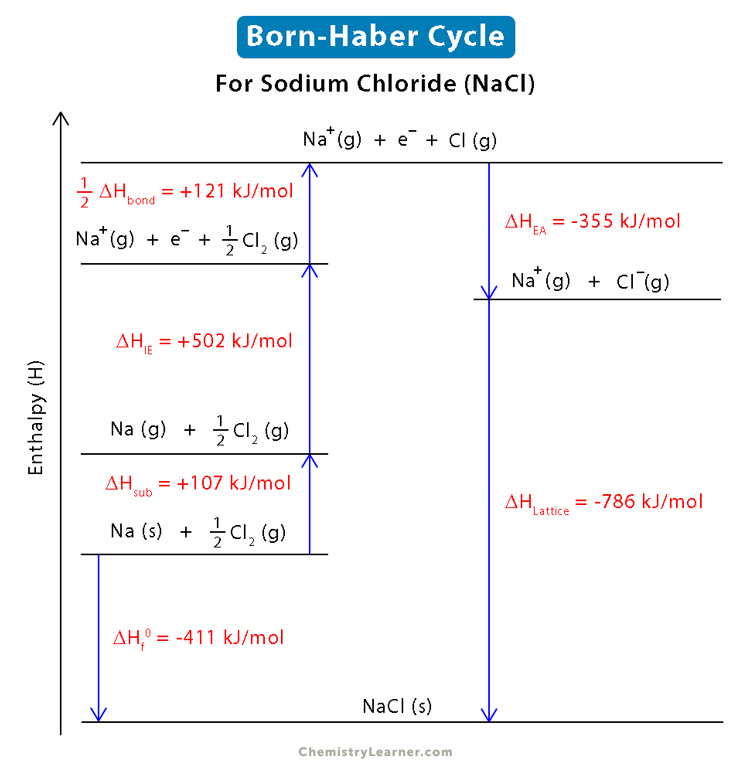
The Born-Haber cycle takes advantage of the enthalpy change to determine the lattice energy of ionic compounds indirectly. It utilizes the known thermodynamic quantities like ionization energy and electron affinity. Let us understand how to use Born-Haber cycle to determine the lattice energy of sodium chloride (NaCl) [1 – 4].
Heat of Formation
Formation involves the reaction between the elements in their standard states to form the ionic compound. The heat of formation is the enthalpy change when forming a compound from its elements. Usually, this value is negative as ionic compounds are stable. It also indicates that the process of formation is an exothermic one.
The heat of formation (ΔHf0), also known as enthalpy of formation, is 411 kJ/mole for sodium chloride.
Na (s) + ½ Cl2 (g) → NaCl (s) ΔHf0 = -411 kJ/mol
The formation of ionic sodium chloride from solid sodium metal and gaseous chlorine is not a single-step process. Instead, it goes through several steps. Energy changes in each step except the lattice energy can be experimentally measured. These steps are given below.
Step 1: Sublimation Energy and Disassociation Energy
The elements involved in the reaction should be in their gaseous forms. Atomization is the formation of gaseous atoms from their naturally occurring elements. Metals in nature occur as single atoms. On the other hand, nonmetals exist in polyatomic form. Hence, energy is added to nonmetals to disassociate into atoms.
- A solid sodium atom undergoes sublimation to form a gaseous atom by absorbing energy. Sublimation energy is the energy required to change the phase of an element from solid to gas. The sublimation energy (ΔHsub) of a solid sodium atom is 107 kJ/mol.
Na (s) → Na (g) ΔHsub = +107 kJ/mol
- Diatomic gaseous chlorine breaks into two individual atoms by absorbing energy. Dissociation energy is the energy required to break apart a compound. The chlorine molecule’s disassociation energy (ΔHbond) is 242 kJ/mol. Each chlorine atom absorbs half of this bond energy.
½ Cl2 (g) → Cl (g) ½ ΔHbond = +121 kJ/mol
Step 2: Ionization Energy and Electron Affinity
Ionization involves the removal of electrons from the gaseous metal to form an ion. In order to accomplish this, energy must be added to the gaseous atom. Ionization energy is the energy required to remove an electron from a neutral atom or ion. On the other hand, energy is released by a gaseous nonmetal atom when electrons are added to form an ion. Electron affinity is the energy released when an electron is added to a neutral atom or an ion.
- A gaseous sodium atom absorbs 502 kJ/mol of energy (ΔHIE) and releases one electron to form a gaseous sodium ion.
Na (g) → Na+ (g) + e– ΔHIE = +502 kJ/mol
- Each gaseous chlorine atom releases 355 kJ/mol of energy (ΔHEA) and absorbs one electron to form a gaseous chlorine ion.
Cl (g) + e– → Cl– (g) ΔHEA = -355 kJ/mol
Step 3: Lattice Energy
The last step is forming the ionic compound from its constituent gaseous ions. The gaseous sodium ion and gaseous chloride ion combine to form a solid sodium chloride molecule and release energy (exothermic) equivalent to the lattice energy (ΔHLE), which we shall determine.
Na+ (g) + Cl–(g) → NaCl (s) ΔHLE = ?
Determining Lattice Energy from Hess’ Law
From Hess’s Law, the overall change in enthalpy of a process can be determined by breaking the process down into steps and then adding the enthalpy changes of each step. So, summing the enthalpy change of all the steps from 1 to 3 gives the enthalpy of formation of solid crystalline sodium chloride from sodium and chlorine in their natural forms of solid and gas, respectively. It should be equal to the experimentally measured enthalpy of formation of solid sodium chloride.
Equation
ΔHf0 = ΔHsub + ½ ΔHbond + ΔHIE + ΔHEA + ΔHLE
Or, ΔHf0 – (ΔHsub + ½ ΔHbond + ΔHIE + ΔHEA + ΔHLE) = 0 ( => ΔHcycle = 0)
Or, -411 kJ/mol – (107 kJ/mol + 121 kJ/mol + 502 kJ/mol – 355 kJ/mol + ΔHLE) = 0
Or, -411 kJ/mol – 375 kJ/mol – ΔHLE = 0
Or, ΔHLE = -786 kJ/mol
How to Draw Born Haber Cycle [1,5]
- Draw a vertical axis denoting the enthalpy axis.
- Draw a horizontal line denoting the energy level of the ionic compound.
- Construct and connect the various energy levels involved in the abovementioned steps with increasing energies.
- First, construct and connect the levels that require energy on the left.
- Then, construct and connect the levels that release energy on the right.
- This diagram will lead to a closed-loop
Using the method described above, let us look at the Born-Haber cycle diagram for sodium chloride.
In this way, the Born-Haber cycle can be constructed for other ionic compounds like lithium fluoride (LiF), calcium fluoride (CaF2), potassium chloride (KCl), magnesium chloride (MgCl2), and magnesium oxide (MgO).
whao ..amazing ..everyone should learn from this
This notes are pricise and concise but a carry a lot of deep insights. thank you
This is an important information . If so more about covalent bonding attach it