Heavy Water
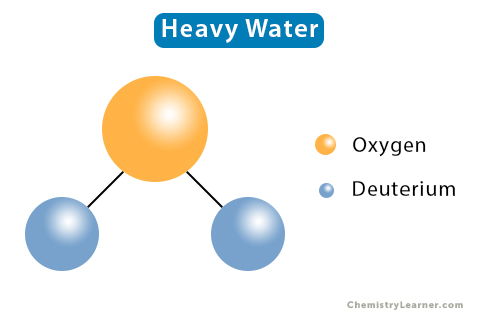
Regular water comprises two atoms of hydrogen and one atom of oxygen. Heavy water is a form of water that contains a deuterium (D or 2H1) atom instead of a hydrogen (1H1) atom. Deuterium is a stable isotope of hydrogen. Thus, the chemical composition of heavy water is two atoms of deuterium and one atom of oxygen with the chemical formula D2O [1-4].
Unlike hydrogen, whose nucleus consists of one proton, deuterium’s nucleus consists of one proton and one neutron. Thus, its atomic mass is twice that of hydrogen. The presence of deuterium gives heavy water different nuclear, chemical, and physical properties than ordinary water.
Heavy water was first produced in 1932, a few months after discovering deuterium.
Preparation
Heavy water is present in natural water. One water molecule for every twenty million water molecules is heavy water. It can be isolated using electrolysis and distillation [1-5].
1. Electrolysis
Hydrogen is liberated significantly faster than deuterium upon electrolysis of water. As a result, H2O bonds break more easily than D2O bonds. When the electrolysis is carried out, just a tiny amount of water remains, and the residue is pure D2O.
2. Distillation
There is a slight difference in the boiling points of water (100 ˚C) and heavy water (101.42 ˚C). This difference allows one to prepare using fractional distillation. As a result, the lighter portion (water) distills first, leaving behind the heavier part (heavy water).
Uses and Applications [5]
Nuclear Reactor
Heavy water finds a significant application in nuclear reactors. A moderator is used to slow down the neutrons and carry out fission reactions effectively during nuclear fission. Heavy water is one of the two moderators – the other being graphite. Unlike ordinary water, heavy water does not absorb neutrons. It slows down, which increases the fission reaction rate, thus enabling a sustained chain reaction.
Other
Aside from nuclear reactors, heavy has a few other applications. In particular, it is used:
- As a tracer, to study respiration and photosynthesis
- For metabolic rate testing in physiology and biology
- In NMR (nuclear magnetic resonance) spectroscopy, it observes the magnetic fields around the nuclei of atoms.
- For the preparation of tritium, used in controlled nuclear fission reactions.
- For the preparation of isotopologues of many organic compounds using deuterium.
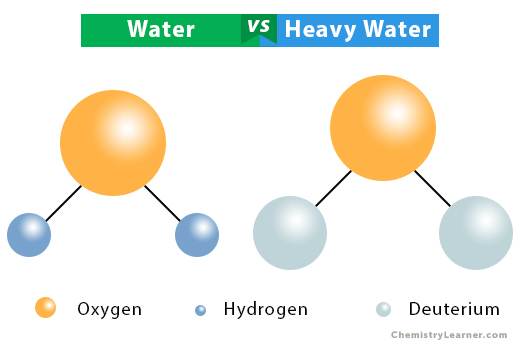
Water vs. Heavy Water
Both water and heavy water have similarities and differences in physical and chemical properties [5].
Heavy water is a colorless, odorless, tasteless, moveable liquid, similar to regular water. It has a lower dielectric constant than water. As a result, ionic chemicals dissolved in heavy water are less soluble than in water.
Heavy water can take part in any chemical reaction that water can take part. However, it responds more slowly than water. It has a lower reactivity than water because the O-D bond has higher dissociation energy than the O-H bond.
The following table gives the difference between the two.
| Water | Heavy Water | |
| Constituents | Two hydrogen and one oxygen atoms | Two deuterium and one oxygen atoms |
| Chemical formula | H2O | D2O |
| Molar mass | 18 g/mol | 20.0276 g/mol |
| Freezing point | 0 ˚C | 3.82 ˚C |
| Boiling point | 100 ˚C | 101.42 ˚C |
| Density | 0.99704 g/m3 at 25 ˚C | 1.106 g/m3 at 25 ˚C |
| Heat of vaporization | 40.66 kJ/mol | 41.61 kJ/mol |
| Heat of fusion | 6.01 kJ/mol | 6.28 kJ/mol |
| Use | Essential for drinking, cleaning, and forms a component of the human body | Used in nuclear reactors and for studying chemical and biological processes |
FAQs
Ans. Heavy water is drinkable in a small quantity without causing any harm to the body.
Ans. If 50% of regular water in the human body is replaced with heavy water, then it can be fatal.
Ans. Heavy water is a better moderator than light water because, compared to light water, it does not capture thermal neutrons.
Ans. Because of its capability of converting 238U into 239Pu by capturing a neutron, scientists during World War II realized that heavy water could be used in this way to make nuclear weapons.