Exceptions to the Octet Rule
The octet rule is applied to draw the Lewis structure of many stable atoms, molecules, and ions. It can accurately predict the molecular formula and structure of covalently bonded molecules. According to this rule, atoms tend to have eight electrons in their outermost or valence shell by losing, gaining, or sharing electrons. However, not all species follow the octet rule. There are three exceptions to it [1-4].
What are the Exceptions to the Octet Rule [1-7]
1. Odd Number of Electrons
Usually, molecules containing s- and p-block elements have an even number of electrons (ex. H2O, CO2, and NH3). However, a few molecules are composed of p-block elements and consist of an odd number of electrons. These classes of compounds are known as free radicals. Examples are nitric oxide (NO), nitrogen dioxide (NO2), and chlorine dioxide (ClO2).
Consider NO, whose Lewis dot structure is shown below. It has 11 electrons (5 from N and 6 from O) in its valence shell.
It is evident from the image that O has 8 electrons in the valence shell and fulfills the octet rule. However, N with 7 electrons in its valence shell does not satisfy the octet rule.
2. Expanded Octet
There are some molecules or ions whose central atom can hold more than 8 electrons in its valence shell. Such molecules are known as hypervalent molecules. This property is observed for elements in the third period or beyond, particularly non-metals. These elements have d-subshell that can occupy up to 10 electrons. Sulfur, phosphorous, silicon, and chlorine are a few elements that have expanded octet. Examples of hypervalent molecules are sulfur hexafluoride (SF6), phosphorous pentachloride (PCl5), chlorine trifluoride (ClF3), xenon tetrafluoride (XeF4), sulfate (SO42-) ion, phosphate (PO43-) ion, and triiodide (I3–) ion.
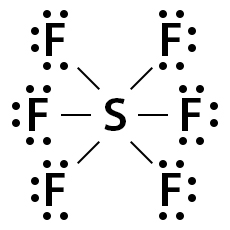
Consider SF6, whose Lewis dot structure is shown below. Its valence shell consists of 48 electrons (6 + (7 x 6) = 48). Sulfur makes a single bond with all six fluorine atoms, thereby completing fluorine’s octet. However, S has 12 electrons around it instead of 8.
Sulfur uses its p- and d-shells to accommodate the 12 electrons. Such a type of molecular structure where the valence shell can take more than 8 electrons is known as an expanded octet.
3. Incomplete Octet
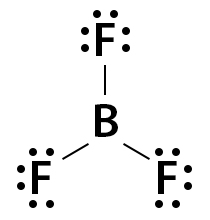
Lighter s- and p-block elements can form compounds with less than 8 valence electrons and hence, have incomplete octets. Hydrogen, lithium, beryllium, and boron have fewer electrons to complete the octet. Helium has 2 electrons in its valence shell but is an inert gas. It does not form a bond with other atoms. Some common examples of incomplete octet molecules are boron trifluoride (BF3), beryllium chloride (BeCl2), and beryllium hydride (BeH2). Boron (B) has 3 valence electrons, and fluorine (F) has 7. Hence, B forms three single bonds with F, as shown below. The number of valence electrons in BF3 is 24 (3 + (3 x 7)).
With 8 electrons in its octet, F satisfies the octet rule. However, B has 6 valence electrons. Hence, BF3 does not follow the octet rule.
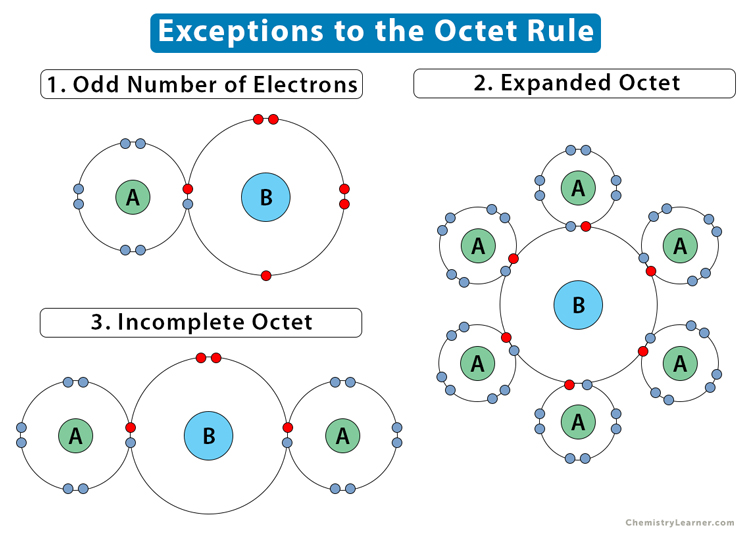
The following image summarizes the exceptions to the octet rule.
FAQs
Ans. The oxygen molecule is not an exception to the octet rule. The oxygen atom has 6 valence electrons and tends to gain or share 2 electrons to complete its octet.
Ans. No, F2 is not an exception to the octet rule. Fluorine has 7 valence electrons and shares 1 electron with another atom to complete its octet.
Ans. No, O3 or ozone is not an exception to the octet rule.