Nitration
Nitration is the process of adding a nitro group into an organic compound like aromatic compound, alcohol, glycol, glycerine, aromatic amine, and paraffin. Most compounds are nitrated by an ionic mixture of strong acids containing nitric acid and sulfuric acid. Sulfuric acid is used because nitric acid is a slow electrophile. The presence of sulfuric acid accelerates the speed of the reaction, and hence, it acts as a catalyst [1-4].
Nitration Examples
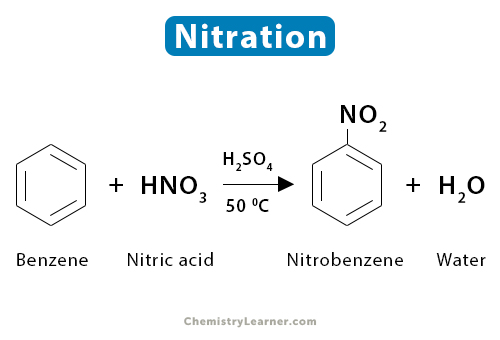
1. Benzene
When an aromatic compound like benzene and toluene is nitrated, the reaction occurs through electrophilic aromatic substitution. Benzene (C6H6) is rich in electrons. The electrophilic nitronium ion (NO2+), present in the nitric acid-sulfuric acid mixture, readily attacks benzene at a temperature not exceeding 50 ˚C, producing nitrobenzene (C6H5NO2). The reaction is accompanied by the loss of a water molecule [1-4].
C6H6 + HNO3 → C6H5NO2 + H2O
Since sulfuric acid is the catalyst, it is not shown in the reaction. There is also a possibility of forming 1,3-dinitrobenzene.
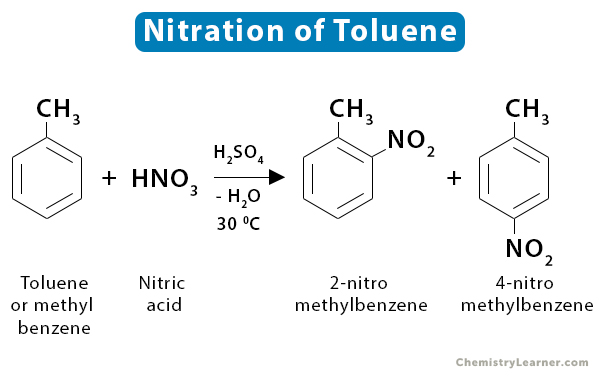
2. Methyl Benzene
Methyl benzene or toluene (C6H5CH3) reacts faster than benzene because the methyl group tends to push the electrons towards the ring. The increasing electron density makes the ring more attractive to the attacking reagent. The reaction is carried out at a lower temperature (~30 ˚C) and produces two isomers: 2-nitromethylbenzene and 4-nitromethylbenzene [2,3].
C6H5CH3 + HNO3 → C6H4CH3NO2 + H2O
Just like benzene, there is a possibility of dinitrobenzene formation.
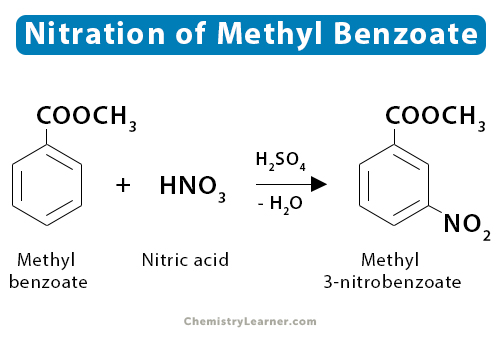
3. Methyl Benzoate
Nitration of methyl benzoate gives methyl-3-nitrobenzoate. The balanced equation is shown below.
C6H5COOCH3 + HNO3 → C6H4COOCH3NO2 + H2O
4. Bromobenzene
Nitration of bromobenzene yield 4-nitrobromobenzene.
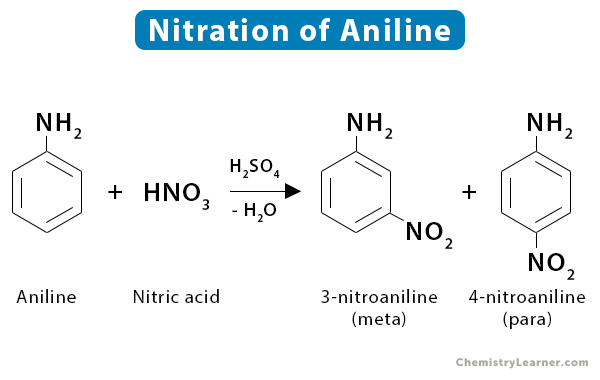
5. Aniline
Nitration of aniline yields meta- and para- nitration products.
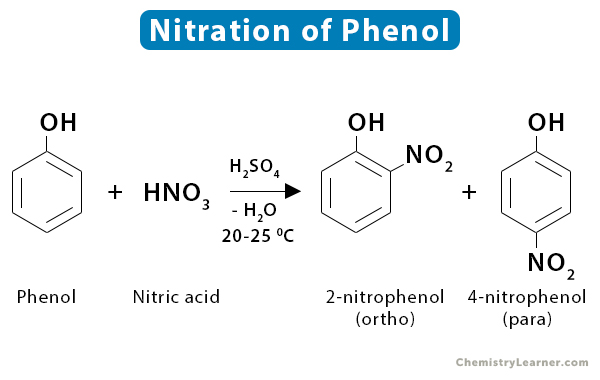
6. Phenol
Nitration of phenol at 25 ˚C yields a mixture of ortho and para nitrophenols.
7. Benzaldehyde
Nitration of benzaldehyde yields meta-nitrobenzaldehydes.
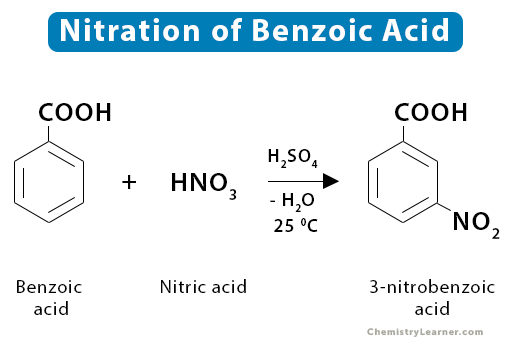
8. Benzoic Acid
Nitration of benzoic acid yields 3-nitrobenzoic acid.
Nitration of Mechanism
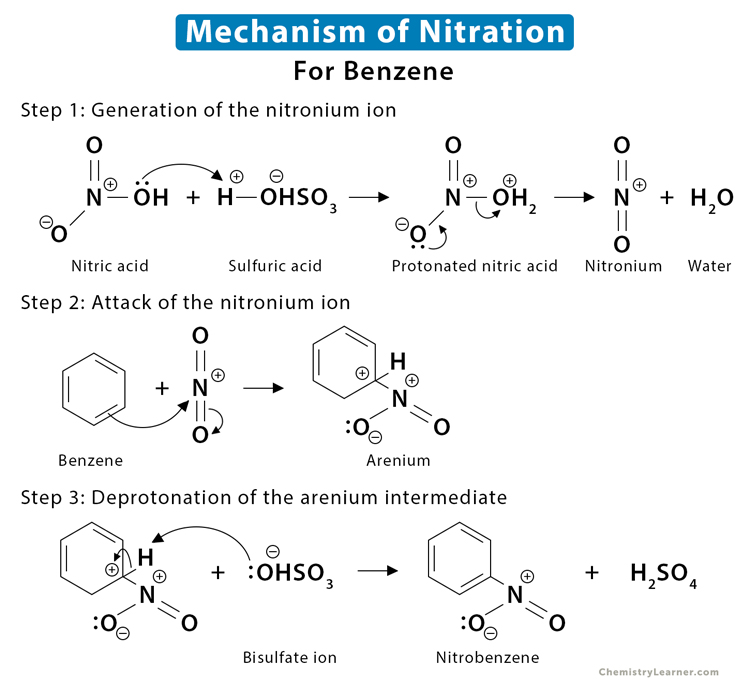
Since most of the examples listed above are benzene derivatives, we shall discuss the mechanism of benzene nitration. The nitronium ion (NO2+) present in the mixture is the active species that attacks the organic compound to form an unstable complex. The nitrated hydrocarbon is formed when a proton is removed from the complex [1-4].
Step 1: Generation of the nitronium ion
The reaction starts with the protonation of the hydroxy (OH–) group in nitric acid (HNO3) by sulfuric acid (H2SO4). When OH– is protonated, a molecule of water (H2O) is formed. Since water is a good leaving group, it is released immediately to give the highly reactive nitronium ion (NO2+). The H2SO4 is converted to a bisulfate (HSO4–) ion.
Step 2: Attack of the nitronium ion
The electron-rich benzene attacks the nitronium ion to give a carbocation intermediate, forming a C-N bond and breaking the C-C and the N-O bonds.
Step 3: Deprotonation of the carbocation intermediate
It is a relatively fast step resulting in an acid-base reaction. The bisulfate ion HSO4– removes a proton from the carbon bearing the nitro group, breaking the C-H bond and reforming the C=C pi bond to restore the aromaticity.
Nitration Reaction Uses
There are many significant applications of nitration in industry. The most important ones are the large-scale production of nitroaromatic compounds such as nitrobenzene. Nitroaromatic compounds are of considerable use as intermediates in the synthesis of pharmaceuticals, plastics, dyes, and insecticides. Nitroaliphatics are used as solvents and synthons in organic synthesis. Nitration reactions are mainly used for the production of explosives. For example, it converts toluene to TNT (2,4,6-trinitrotoluene) [5].