Electron Shielding
What is Electron Shielding
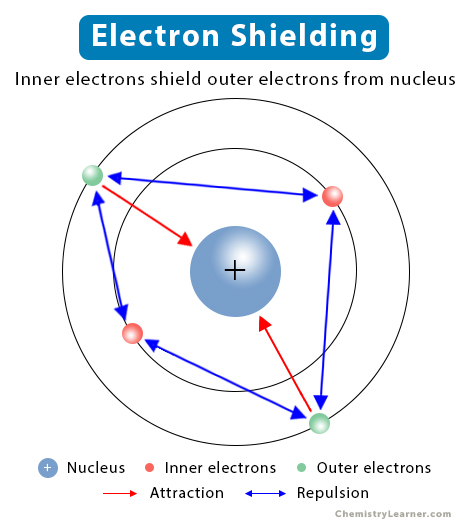
In a multi-electron atom, the orbiting electrons are attracted to the nucleus by electrostatic forces. However, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. In other words, the inner electrons shield the outer electrons from the nucleus. This phenomenon is known as electron shielding. The higher the number of electrons, the greater the shielding will be. Shielding affects the ionization energy of the atom. It explains why the valence electrons are easy to remove than the inner electrons [1-4].
Electron Shielding and Ionization Energy
A hydrogen atom has no shielding effect since it has only one electron. Therefore, using Coulomb’s law, one can easily calculate the electrostatic force of attraction. As the number of electrons increases, shielding becomes more pronounced. Due to repulsion among the electrons, the valence electrons feel a weak electrostatic attractive force. Hence, it is easy to remove an electron from the outermost orbit. Ionization energy is the energy required to remove a valence electron from its orbit. The higher the shielding, the lower the ionization energy [1,2].
It is challenging to calculate the magnitude of electron shielding. For this reason, an effective nuclear charge is used.
Electron Shielding and Atomic Orbital
Generally, the inner electrons shield the outer electrons. However, there is a shielding effect among the electrons in the same principal energy level (n). It is known that the electrons reside in atomic orbitals, which have different shapes. For example, the s-orbital has a spherical shape, whereas the p-orbital has a dumbbell shape. The s-orbital is more penetrating and closer to the nucleus than the p-orbital. As a result, the attractive force on the p-electrons reduces. Therefore, the s-orbital can shield all three p-orbitals, not the other way around [1,4,5].
In general, wider orbitals provide better shielding. The order of decreasing shielding capacity is as follows.
s > p > d > f
Examples [1,2,5]
- Lithium has three electrons – two in 1s-orbital and one in 2s-orbital. The 1s-electrons shield the 2s-electron. Hence, lithium has two shielding electrons, making it easy to remove the only valence electron.
- Magnesium has two valence electrons in the 3s-orbital. The inner electrons shield them. On the other hand, aluminum has three valence electrons – two in the 3s-orbital and one in the 3p-orbital. The 3p-electron is shielded by the two 3s-electrons and the inner electrons. Therefore, it is easier to remove the outermost electron from aluminum than magnesium. As a result, aluminum has lower ionization energy than magnesium.
- The electron configuration of sodium is [Ne] 3s1, and cesium is [Xe] 6s1. It means sodium has two electron shells inside, and cesium has five. Although cesium has more protons than sodium, one would expect a higher electrostatic force in the former than in the latter. However, cesium also has more electrons, and shielding is higher than sodium. As a result, the electrostatic force is reduced, making it easier to remove the valence electron.