Alkene Reactions
What is an Alkene Reaction? (1-4)
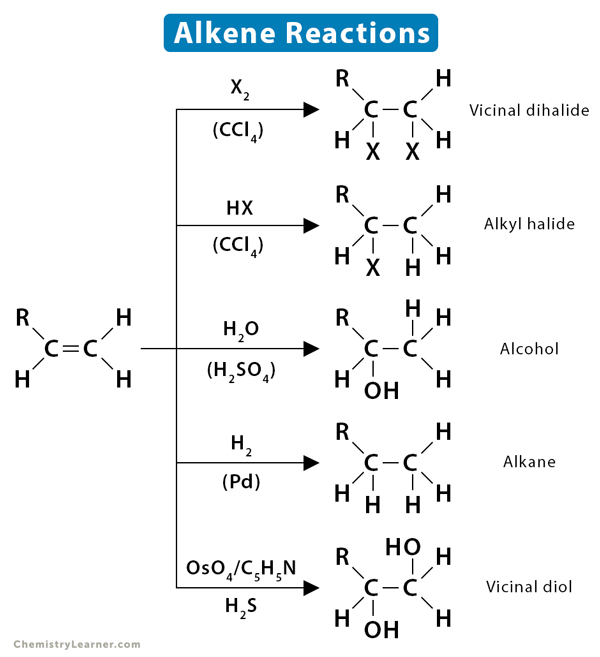
Alkenes are hydrocarbons that contain one or more double bonds. Their double bond consists of a sigma (σ) bond and a pi (π) bond. The carbon-carbon π bond is formed by side-by-side overlapping. It is relatively weak and exhibits high reactivity. The bond can be easily broken, and reagents can be added to carbon. The π-electrons also make the carbons atom electron-rich and attract an electrophile. Thus, alkenes can undergo a large variety of reactions. Alkenes can be used to synthesize a wide variety of compounds, such as halide, alcohol, ethers, and alkanes.
Types of Alkene Reaction (1-4)
1. Addition
The most common reaction for an alkene is the addition reaction. It involves the addition of two atoms to the molecule or compound. As already stated, breaking the π-bond of alkenes is relatively more straightforward than the σ-bond. So, one π-bond gets converted to two σ-bonds during the addition reaction, attaching to the incoming atoms.
In the addition reaction, π-bond is necessary. Unlike alkenes, alkanes do not undergo an addition reaction because they do not have a π-bond. They can participate in substitution reactions.
General Formula for Addition Reaction of Alkene
To understand the addition reaction, let us take an alkene R1CH=HCR2, which reacts with a hydrogen halide HX, yielding an alkyl halide, R1CHBX-H2CR2. The reaction is as follows:
R1CH=HCR2 + HX → R1CHBX-H2CR2
Types of Addition Reaction of Alkene
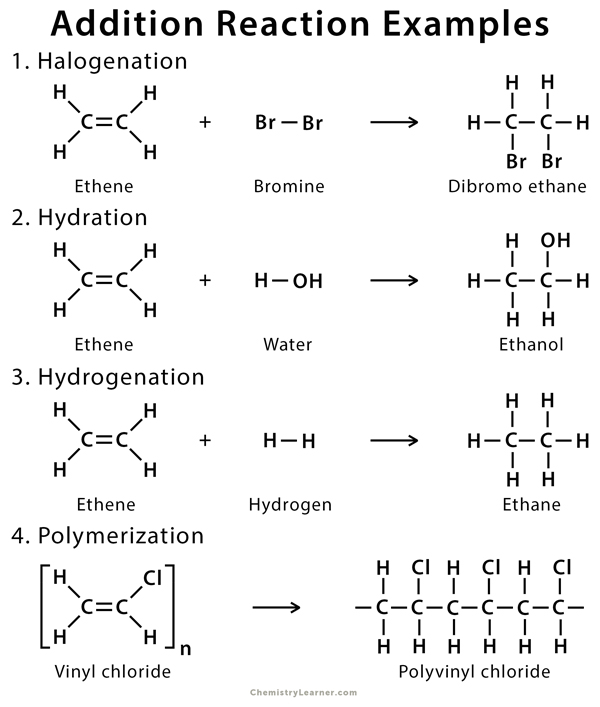
1.1 Hydrogenation
Hydrogenation is the addition of hydrogen (H2) to the π-bond of alkenes, producing an alkane. The reaction must be catalyzed by metals such as Pd, Pt, Rh, and Ni.
Example
1. Ethene (C2H4) converts into ethane (C2H6) by reacting with hydrogen at 150 ͦC.
C2H4 + H2 → C2H6
2. Cyclohexene (C6H10) reacts with hydrogen in the presence of palladium (Pd) catalyst to produce cyclohexane (C6H12).
C6H10 + H2 → C6H12
1.2 Halogenation
Halogenation of alkene is an addition reaction where a halide, such as Cl2 or Br2, gets attached to the alkene breaking the double bond between the carbon atoms. The resulting product is a vicinal (neighboring) dihalide.
Example
1. Ethene (CH2 = CH2) reacts with chlorine (Cl2) in the presence of carbon tetrachloride (CCl4), producing dichloroethane (CH2Cl – ClCH2).
CH2 = CH2 + Cl2 → CH2Cl – ClCH2
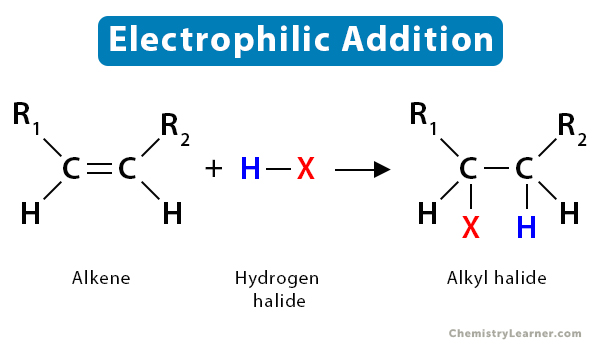
1.3 Hydrohalogenation
In hydrohalogenation of alkenes, the double bond between carbon atom breaks, followed by the electrophilic addition of a hydrogen atom and halogen. Following Markovnikov’s rule, the halide gets attached to the more substituted carbon. The resultant product is a haloalkane or alkyl halide.
Examples
1. Ethene (CH2=CH2) reacts with HBr in the presence of CCl4, producing bromoethane or ethyl bromide (CH3CH2Br).
CH2=CH2 + HBr → CH3CH2Br
2. Propene (CH3CH=CH2) reacts with hydrogen chloride, producing 2-chloropropane or 2-propyl chloride (CH3CHClCH3) as the major product and 1-chloropropane (CH3CH2CH2Cl) as the minor product.
CH3CH=CH2 + HCl → CH3CHClCH3 (major product, more stable) + CH3CH2CH2Cl (minor product, less stable)
Here, the two products CH3CHClCH3 and CH3CH2CH2Cl are constitutional isomers.
1.4 Hydration
The addition of water to alkenes is known as hydration. In this reaction, the π-bond of alkene and OH bond in water breaks, producing alcohol. The hydration process usually makes many simple alcohols of alkenes in the presence of an acid catalyst.
Example
Ethene (CH2=CH2) reacts with water in the presence of a strong acid such as sulfuric acid (H2SO4) to produce ethanol (CH3CH2OH).
CH2=CH2 + H2O → CH3CH2OH
1.5 Polymerization
In this addition reaction, an alkene reacts with itself to form a high‐molecular‐weight compound composed of repeating units of the original compound. Here, the alkene acts as a monomer, producing polymer by joining its repeated units to give a single product.
Example
During the polymerization of ethene (CH2=CH2), thousands of ethene molecules join together to make poly(ethene) or polythene (-CH2-CH2-CH2– CH2– CH2-)n.
[CH2=CH2]n → (-CH2-CH2-CH2-CH2-CH2-)n
2. Oxidation
Alkenes can easily undergo oxidation under the influence of oxidizing agents, such as potassium permanganate. The products formed depend on the reaction conditions. At low temperatures and low concentrations of oxidizing reagents, alkenes tend to form glycols.
Example
Ethene (CH2=CH2) reacts with 1-4 % potassium permanganate (KMnO4) solution to produce ethylene glycol (HOCH2-CH2OH), manganese dioxide (MnO2), and potassium hydroxide (KOH).
3C2H4 + 2KMnO4 + 4H2O → 3HOCH2-CH2OH + 2MnO2 + 2KOH
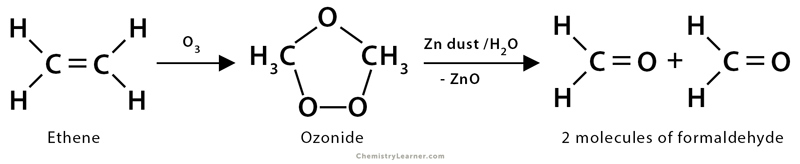
3. Ozonolysis
Ozonolysis is the oxidation of alkenes by ozone. Here, both the σ and π bonds of the alkenes get destructed. Ozone (O3) has a dipolar ion structure which first adds to the π-bond of alkene to form a highly unstable molozonide structure and then gets rearranged to another structure by breaking of C-C σ-bond to give ozonide. It is further cleaved under a reductive atmosphere using Zn dust/H2O to form corresponding carbonyl compounds, like aldehydes and ketones. This oxidative cleavage of an alkene double bond generally accomplishes good yield.
Example
1. Ethene (CH2=CH2) undergoes ozonolysis, yielding two molecules of formaldehyde (HCHO). An ozonide is formed as an intermediate product. In the presence of zinc dust and H2O, the ozonide gets transformed into formaldehyde.
CH2=CH2 + O3 → Ozonide
Ozonide → CH2O + CH2O
The ozonolysis reaction is often used to find the position of the double bond in an alkene molecule. For instance, the isomers of C4H8, 2-butene, and 1-butene, can be distinguished via oxidative cleavage. By identifying the products, one isomer can be distinguished from another, and the position of the bonds in the original compound can be determined.
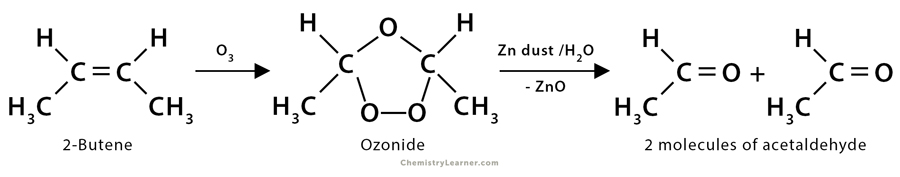
2. Ozonolysis of 2-butene: Here, 2-butene (CH3CH=CHCH3) yields two acetaldehyde molecules (CH3CHO) after undergoing ozonolysis.
CH3CH=CHCH3 + O3 → Ozonide
Ozonide → 2CH3COH + H2O + ZnO
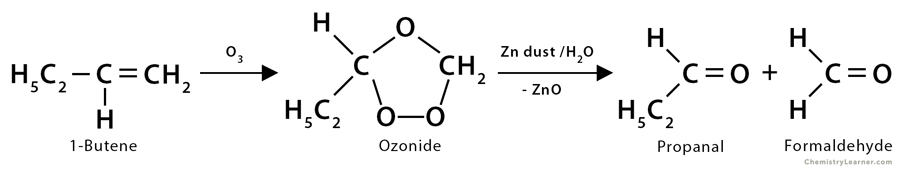
3. Ozonolysis of 1-butene: In the ozonolysis reaction of 1-butene (CH3CH2CH=CH2), it produces one molecule of propanal (CH3CH2CHO) and one molecule of formaldehyde (HCHO).
CH3CH2CH=CH2 + O3 → Ozonide
Ozonide → CH3CH2COH + CH2O
4. Other Reactions of Alkene
4.1 Oxymercuration Demercuration
In this reaction, an alkene reacts with mercuric acetate [Hg(OAc)2] in the presence of an oxygen nucleophile, such as water or alcohol, to form an organomercury intermediate, such as hydroxyl or ethoxy mercurial compounds. The bond present between the carbon and mercury of the intermediate product gets converted to a carbon-hydrogen bond after treatment with sodium borohydride (NaBH4). As a result of the overall reaction, the alkene converts into alcohol or ether, depending on the nucleophile used. If the nucleophile is water, the alkene gets converted into alcohol. When the nucleophile is an alcohol, the alkene gets converted into an ether. Thus, the hydroxyl or ethoxy mercurial compounds are further reduced to alcohols or ethers, respectively.
Example
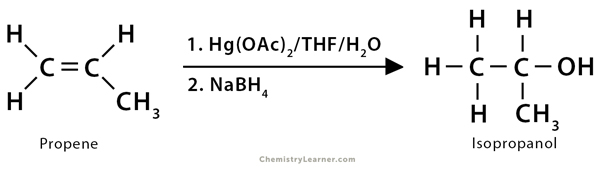
1. Propene (CH3CH=CH2) reacts with water and mercuric acetate (Hg(OAc)2) followed by NaBH4, giving isopropyl alcohol or 2-propanol (CH3CHOHCH3). The overall reaction is the electrophilic addition of water to the alkene following Markovnikov’s rule.
4.2 Hydroboration-Oxidation
Hydroboration is an oxidation reaction in which alkenes transform into alcohol by reacting with water. It is a two-step reaction. In the first step, diborane (B2H6) is added to form trialkyl boranes. The reaction proceeds in an anti-Markovnikov manner. The hydrogen from B2H6 attaches to the more substituted carbon, and the boron attaches to the least substituted carbon in the alkene double bond. The trialkyl borane is oxidized using an alkaline hydrogen peroxide solution in the next step. It leads to the formation of primary alcohols.
Example
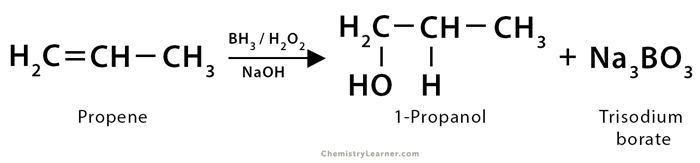
1. In the hydroboration of propene (CH3CH=CH2), diborane (B2H6) is added in the first step to form trialkyl boranes [(CH3CH2CH2)3B]. The next step is the oxidation of trialkyl borane, which is done using an alkaline hydrogen peroxide (H2O2) solution, leading to the formation of 1-propanol (CH3CH2CH2OH) along with trisodium orthoborate (Na3BO3).
4.3 Dihydroxylation
Dihydroxylation is when an alkene is converted into a vicinal diol. The most common and direct process for this oxidation is using a high-oxidation-state transition metal, typically osmium or manganese. The metal is often used as a catalyst, with other oxidizing agents like hydrogen peroxide (H2O2).
Example
1. In the dihydroxylation of 2-butene (CH3CH=CHCH3), it reacts with osmium tetroxide (OsO4) in the presence of hydrogen peroxide (H2O2). The products depend on the type of butane used. If it is cis-2-butene, the products are meso-2,3-butanediol (CH3CH(OH)CH(OH)CH3. On the other hand, if it is trans-2-butene, the products are a racemic mixture of enantiomers.
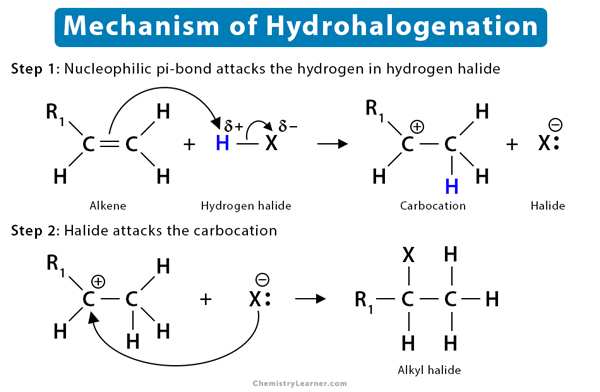
Mechanism of Alkene Reaction (5)
To understand the mechanism of the alkene reaction, let us take the example of the hydrohalogenation reaction. It is an electrophilic addition reaction as an electrophile initially attacks the substrate.
The fundamental mechanism of hydrohalogenation of alkenes is as follows:
- First, the nucleophilic π-bond of the alkene breaks down as it tries to grab the electrophilic H+ from hydrogen halides, such as HBr.
- Then, following Markovnikov’s rule, H+ gets added to the less substituted carbon atom. The more substituted carbon is now deficient in electrons, thus turning into a carbocation.
- Finally, the halide ion (Br–) present in the solution attacks the carbocation, forming alkyl bromide.
FAQs
Ans. Alcohol is the main organic product in the reaction between alkene and borane in tetrahydrofuran.
Ans. The free radical addition reactions of alkenes produce anti-Markovnikov products.
Ans. No, the Grignard reagent does not directly react with an alkene.
Ans. Selenium dioxide (SeO2) is an oxidizing agent generally used in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones.
Ans. The combustion reaction of alkene produces carbon dioxide and water.
References
- Reactions of Alkenes – Crab.rutgers.edu
- Reactions of Alkenes: Addition Reactions – Chemistry.msu.edu
- Reactions of Alkenes: Addition of Hydrogen Halide to Alkenes – Kpu.pressbooks.pub
- Summary of Alkene Reactions – Chem.libretexts.org
- Hydrohalogenation Of Alkenes – Reaction Mechanism – Leah4sci.com