Electron Affinity
What is Electron Affinity
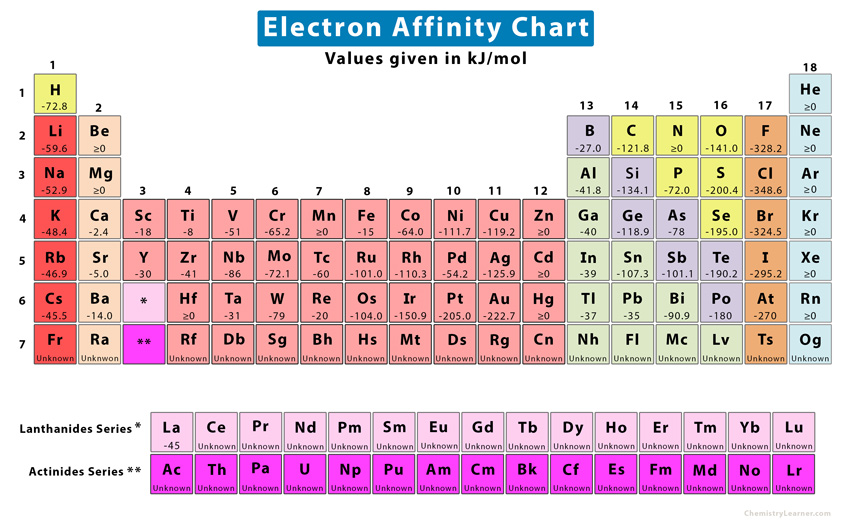
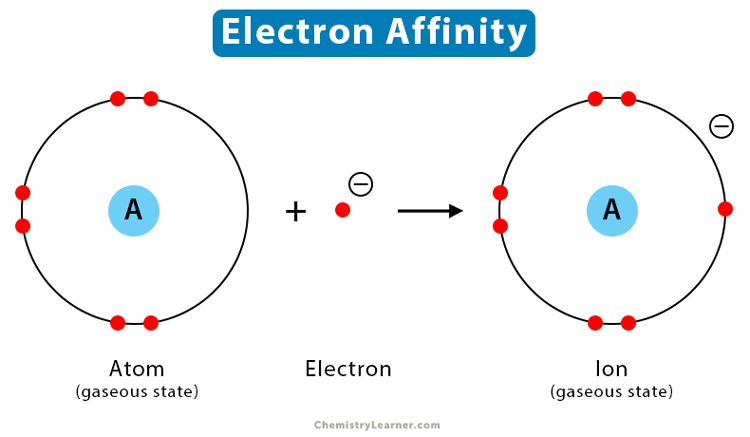
The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. It is the opposite of ionization energy [1-4].
How to Find Electron Affinity
The electron affinity is generally challenging to determine. However, it is measured for atoms in which the molecule is in a gaseous state. The reason is that, in a gaseous state, the atoms are adequately separated, and their energy levels are not affected by other atoms. The Born-Haber cycle is used to determine its value indirectly. The electron affinity is represented by the symbol EA and has the energy unit kJ/mol [1,2].
Electron Affinity Chemical Equation and Example
The chemical equation for electron affinity is as follows [1-4].
X (g) + e– → X– (g)
When an electron approaches a neutral atom, the positively charged nucleus attracts it. The atom transforms into a negatively charged ion or an anion. Consequently, energy is released in this process, making the process exothermic. The stronger the attraction, the higher is the energy released. Since energy is released, the electron affinity values are negative. Higher negative values indicate a stable anion.
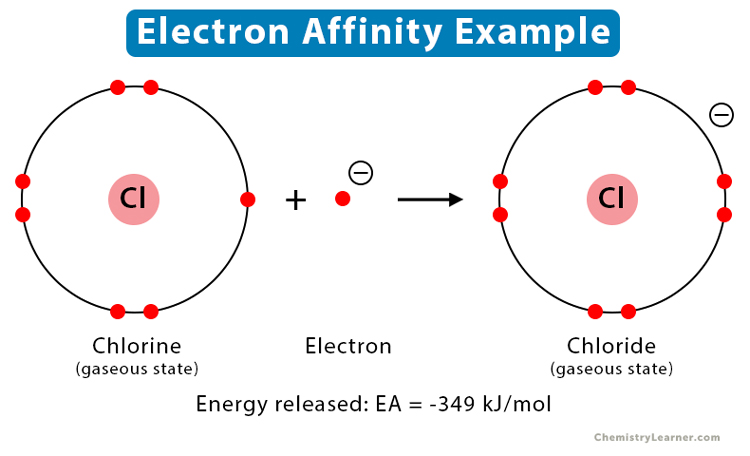
Example: When an electron is added to neutral chlorine (Cl) atom, it transforms into a chloride (Cl-) ion. The energy released is 349 kJ/mol.
Cl (g) + e– → Cl– (g)
Therefore, the electron affinity of chlorine is -349 kJ/mol.
What are the Observed Periodic Trends in Electron Affinity
The elements of the periodic table display a specific trend in electron affinity based on their ability to gain electrons [5-8].
1. Horizontal Trend: Electron Affinity Across a Period
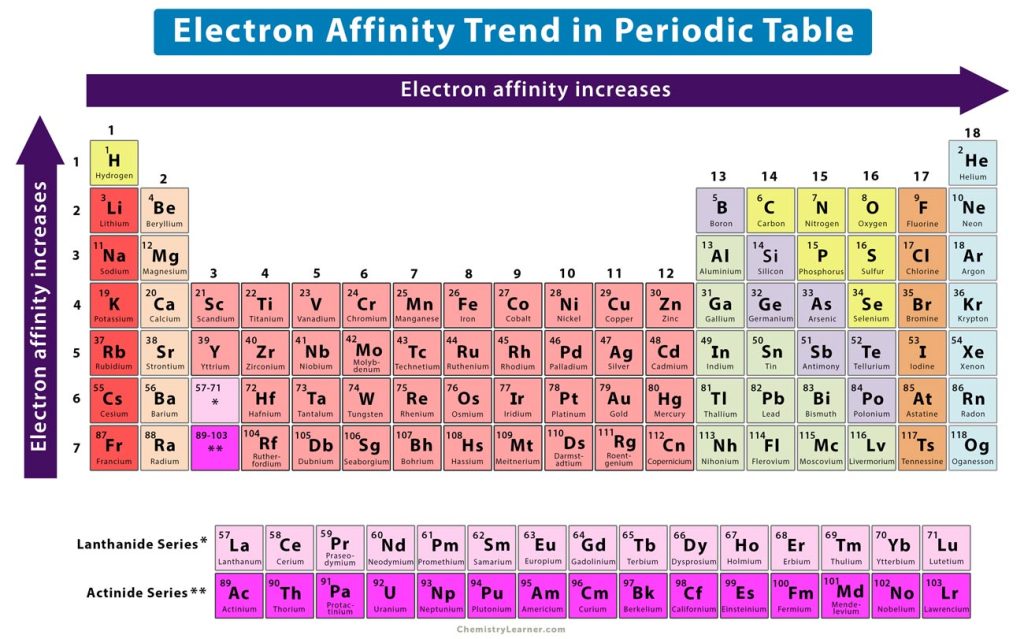
Across a period from left to right, the atomic number of the element increases gradually. As a result, the number of protons increases, thereby increasing the nuclear charge. It means that when an electron approaches an atom, it experiences a stronger attractive electrostatic force. A large amount of energy is released when the atom transforms into an ion. Hence, the electron affinity values are higher. Therefore, the electron affinity increases from left to right across a period, as shown in the image above.
2. Vertical Trend: Electron Affinity Down a Group
Down a group from top to bottom, the atomic number increases suddenly. As a result, the valence electrons occupy higher orbits that are far away from the nucleus. These outer electrons are shielded by the inner electrons and experience a weak electrostatic attraction from the nucleus. When an electron adds to the valence shell of a neutral atom, it is attracted by a weak force. Hence, the energy released is low. Therefore, the electron affinity decreases down a group. In other words, the electron affinity increases from bottom to top in a group, as shown in the image above.
Metals vs. Nonmetals
It is evident from the trend that nonmetals and halogens, which lie on the right of the periodic table, have a higher electron affinity than metals, which lie on the left. The reason is that nonmetals and halogens have nearly complete valence shells and require few electrons to complete their octet. Moreover, they have smaller atomic radii than the corresponding metals in the same period. Therefore, they can easily accept an electron and release large amounts of energy.
On the other hand, metals have fewer protons than nonmetals and halogens. The low nuclear charge does not easily attract an approaching electron. Therefore, metals have low electron affinities. They prefer to donate electrons and absorb energy in that process.
Exceptions
There are a few exceptions to the periodic trend that are discussed below.
Consider the positions of oxygen and fluorine in the periodic table. Both these elements lie above sulfur and chlorine, respectively. However, the electron affinities of oxygen (-142 kJ/mol) and fluorine (-328 kJ/mol) are less than those of sulfur (-200 kJ/mol) and chlorine (-349 kJ/mol), respectively. The reason is that oxygen and fluorine atoms are so small that the orbital electrons are clustered in a tiny space. When a new electron approaches any one of these atoms, it will be repelled by the electron cloud. This repulsion makes the approaching electron less attractive to the atom, and the energy released is reduced.
2. Inert Gases, Beryllium, Calcium, and Nitrogen
The electron configuration of atoms determines whether an atom can achieve stability by donating or accepting electrons. Atoms with a stable electron configuration have less tendency to attract electrons. These include atoms having completely filled valence shells and half-filled or completely-filled subshells in their valence shells. They have low values (nearly zero) of electron affinity. Examples include the following.
- Inert gases have completely filled valence shells
- Beryllium and calcium have filled s-subshells
- Nitrogen has a half-filled p-subshell.
Thus, the factors affecting electron affinity are atomic size, nuclear charge, and stable electron configuration.
Electron Affinity and Electronegativity
Electronegativity and electron affinity are properties of an atom that rely on nuclear properties and the electrostatic attraction between the protons and electrons. The following table gives the difference between the two [7].
| Electronegativity | Electron Affinity | |
|---|---|---|
| Definition | A measure of how much an atom attracts electron pairs in a chemical bond | A measure of an atom’s energy changes when an electron is added to a gaseous atom |
| Unit | Pauling scale | kJ/mol |
| Qualitative or Quantitative | Qualitative | Quantitative |
| Applied to | Single-atom | Atom or molecule |
| Element with the greatest value | Fluorine | Chlorine |
| Element with the lowest value | Cesium and Francium | Mercury |
The electron affinity discussed so far is known as the first electron affinity.
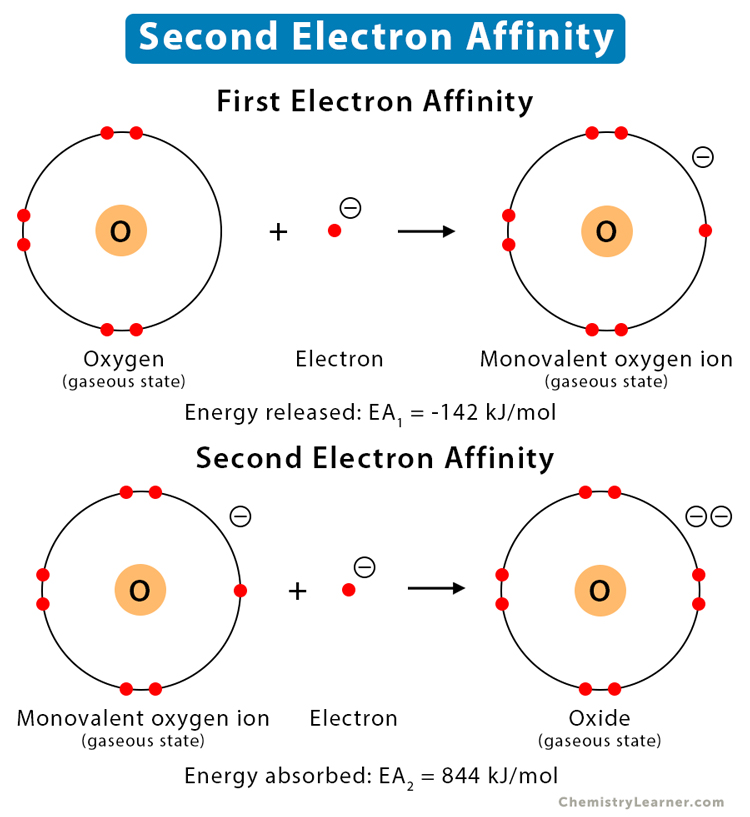
Second Electron Affinity
Until now, we have discussed an electron being added to a neutral atom to form an ion. Now, what happens when an electron is added to an ion. To force an electron into the orbit of an already negative ion, energy is required to overcome the electrostatic repulsion. The energy required to add one electron to one mole of an ion is known as the second electron affinity. Since energy is added to the system, the process is endothermic, and the energy is positive. The result is a divalent anion [1,2].
General Chemical Equation
X– (g) + e– → X2- (g)
Example
When an electron is added to neutral oxygen (O) atom, a monovalent oxygen anion (O–) is formed, and 142 kJ/mol of energy is released. When a second electron is added to the monovalent oxygen (O–) anion, a divalent oxygen anion (O2-) is formed, and 844 kJ/mol of energy goes into the process.
First Electron Affinity: O (g) + e– → O– (g) (EA1 = -142 kJ/mol)
Second Electron Affinity: O– (g) + e– → O2- (g) (EA2 = 844 kJ/mol)
FAQs
Ans. The element with the most negative electron affinity is chlorine, with a value of -349 kJ/mol.
Ans. Mercury has the lowest electron affinity.