What is Hydrogenation
Hydrogenation is an essential chemical reaction for the synthesis of new chemicals. The reaction occurs between a hydrogen molecule and an unsaturated compound, like alkene and alkyne, in the presence of a metal catalyst. By adding hydrogen atoms to a double or triple bond, hydrogenation changes the structure of the molecule. While some amount of energy is required to start the process, the overall reaction is exothermic. The energy of the reactants is more compared to the energy of the product. The bonds formed are more stable than the bonds being broken [1].
General Equation for Hydrogenation Reaction
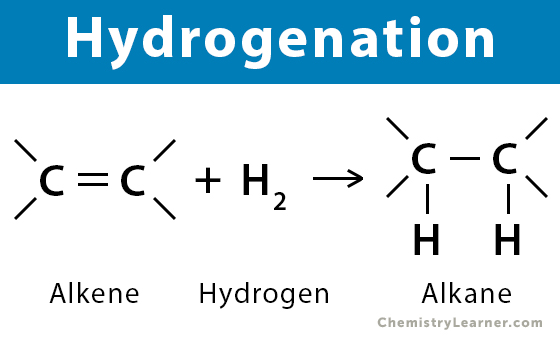
The hydrogenation reaction is an addition reaction in which hydrogen is added to an alkene. Here is the general formula for the hydrogenation reaction.
-CH=CH- + H2 → -CH2-CH2–
Conditions for Hydrogenation
Here are some conditions for hydrogenation.
- One of the reactants must be an unsaturated compound like alkene or alkyne, and the other is a hydrogen molecule
- The reaction is carried out in the presence of a metal catalyst like nickel (Ni), palladium (Pd), platinum (Pt), or rhodium (Rh)
- The temperature of the reaction is maintained at 150 ͦC
Examples of Hydrogenation Reaction
Here are some examples of hydrogenation [1-4].
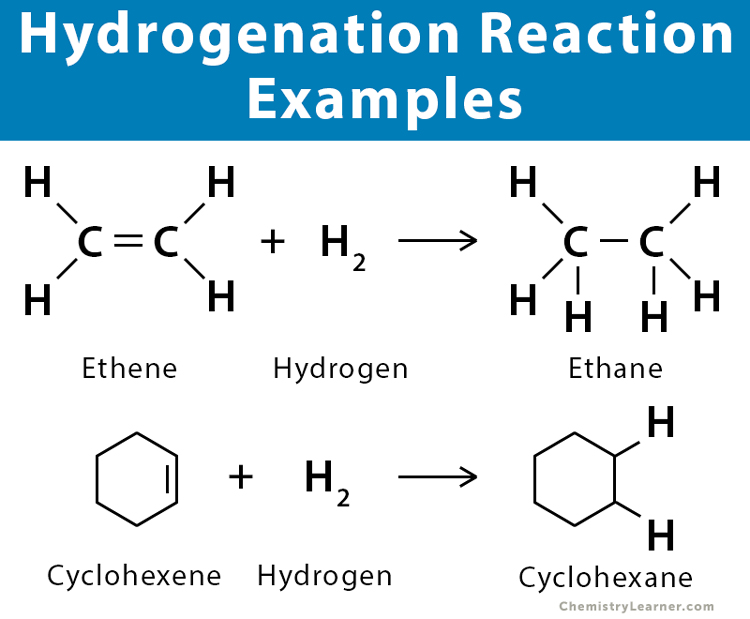
- Alkene: Ethene (C2H4) reacts with hydrogen at 150 ͦC to produce ethane (C2H4).
C2H4 + H2 → C2H6
- Cycloalkene: Cyclohexene (C6H10) converts into cyclohexane (C6H12) by reacting with hydrogen in the presence of palladium (Pd) catalyst.
C6H10 + H2 → C6H12
Heat of Hydrogenation
The heat of hydrogenation of an unsaturated compound is the enthalpy of reaction between a hydrogen molecule and the compound. It is a measure of the stability of carbon-carbon double and triple bonds. Small absolute values denote a stable bond [5].
Examples: The heat of hydrogenation of alkene is -30 kCal mol-1, and that of alkyne is -69 kCal mol-1
Mechanism of Hydrogenation Reaction [4,6]
The following image shows the mechanism of the hydrogenation reaction.
Application of Hydrogenation Reaction [3,7]
Hydrogenation has many applications in the food industry, petrochemical industry, and pharmaceutical industry. It is used to solidify and preserve food and purify raw materials and products.
The most widespread application of hydrogenation is in the production of hydrogenated vegetable oil. Vegetable oil consists of mono and polyunsaturated fats. When the unsaturated C=C bonds are hydrogenated, they are converted into saturated fats with a relatively higher melting point. Therefore, the saturated products are solids or semi-solids. Partially hydrogenated oils can affect heart health because they increase LDL cholesterol and lower HDL cholesterol.
In the petroleum industry, hydrogenation is used in a process called hydrocracking. This process breaks heavy crude’s long hydrogen carbon chains into lighter petroleum products like diesel, gasoline, and jet fuel.