Ion-dipole Forces (Interaction)
What are Ion-dipole Forces
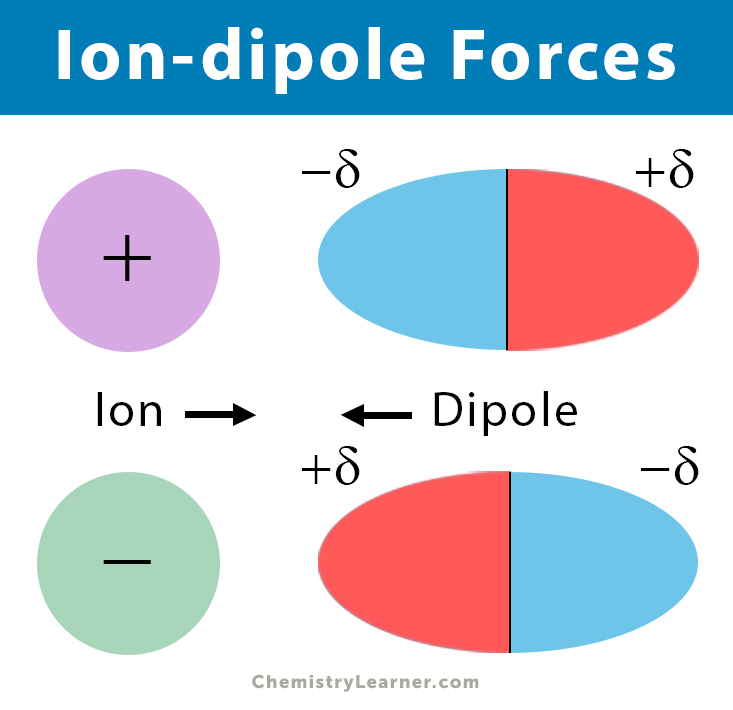
Ion-dipole forces or ion-dipole interactions are the forces of attraction between ions and dipoles. They are typically found in solutions containing ions. Compounds break down into ions in polar solvents and form bonds between themselves and the polar solvent. The forces are electrostatic and occur between the ions and the oppositely charged end of the polar molecule. The positive ions are attracted to the negative ends of the polar molecule, and the negative ions are to the positive ends. Ion-dipole forces are mainly responsible for the dissolution of ionic substances in water [1-8].
Characteristics of Ion-dipole Forces
Here are some facts and characteristics of the ion-dipole forces which can be applied to determine it.
- The ions and dipoles are aligned closer to one another so that the forces are maximum.
- Their strength is proportionate to ion charge or dipole strength.
- They are stronger than the dipole-dipole forces
- They are weaker than the intramolecular ionic and covalent bonds.
Example of Ion-dipole Forces
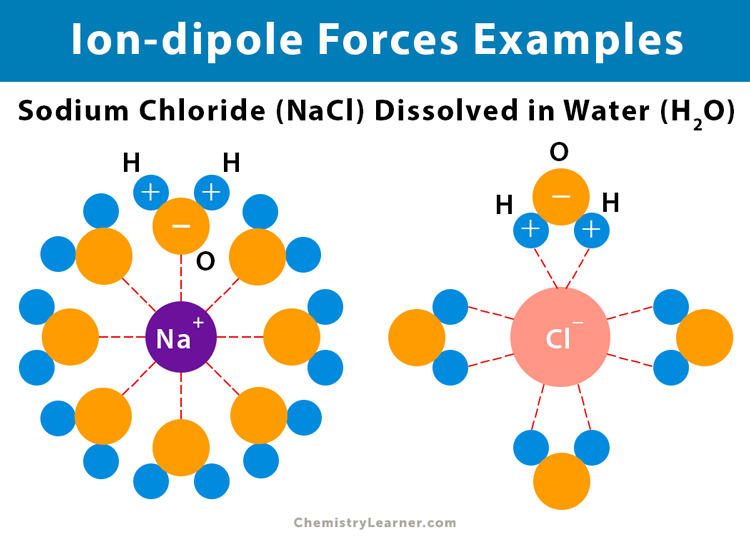
Examples of the ion-dipole forces are the forces between sodium (Na+) and chlorine (Cl–) and a polar water molecule (H2O). Here, the positively charged sodium ion is attracted to the negatively charged oxygen (Oδ-) atom, and the negatively charged chloride (Cl–) ion is attracted to the positively charged hydrogen (Hδ+) atom [1-8].
Ion-induced Dipole Forces
An ion-induced dipole force occurs when an ion interacts with a nonpolar molecule. In this case, the charge on the ion induces polarity in the nonpolar molar molecule. The two interacting species then attract one another and form a weak bond. The strength of this force will depend on the charge on the ion and the polarizability of the molecule. The phenomenon is very similar to dipole-induced dipole force [7,8].
Example: In hemoglobin, the interaction between iron (II) ions (Fe2+) and oxygen molecule (O2) is ion-induced dipole force.