Peptide Bond
What is a Peptide Bond?
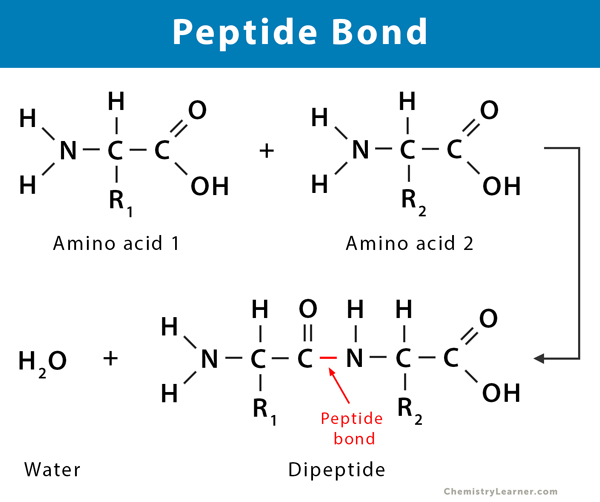
A peptide bond is a covalent chemical bond formed by linking the carboxyl group of one free amino acid molecule to the amino group of another. During this process, a molecule of water is released – a process known as dehydration or condensation. A long chain of amino acids is linked by peptide bonds (CO-NH). It is a strong bond that is commonly found in proteins [1-5].
How are Peptide Bonds Formed?
Here are the steps in the formation of a peptide bond [1-5]:
Step 1: Two amino acid molecules are brought together. The carboxyl group of one amino acid molecule moves towards the amino group of another molecule.
Step 2: The carboxyl group releases the hydroxyl (OH–). Simultaneously, the amino group of the other amino acid molecule releases a proton (H+). The two ions combine to form a molecule of water, which is eliminated from the reaction.
Step 3: A peptide bond is formed when the two amino acids react together.
The newly formed compound is known as a dipeptide because it is formed from two amino acids. Likewise, when three amino acids combine, a tripeptide is formed.
Characteristics of Peptide Bond in a Polypeptide
Here are some properties and features of a peptide bond [1-4].
- It connects two amino acid groups through dehydration synthesis.
- It shows a partial double bond character due to resonance.
- It is strong, kinetically stable, and requires high activation energy to break the bond. Only a long-time exposure to strong acid or base at an elevated temperature can break it. Some specific enzymes, like digestive enzymes, can result in the cleavage.
- The peptides are rigid and planar, which means that the C=O and the N-H bonds lie on the same plane, and the rotation about the peptide bond is restricted. The lack of rotation allows the peptide group to remain fixed in either a cis or trans configuration (stereochemistry), thereby stabilizing protein structure.
- Trans configuration of the peptide group allows less steric hindrances of adjacent amino acid side chains.
- The N-H and C=O bonds are polar, i.e., partially positive H and partially negative O. Hence, different regions of the peptide can form hydrogen bonding.
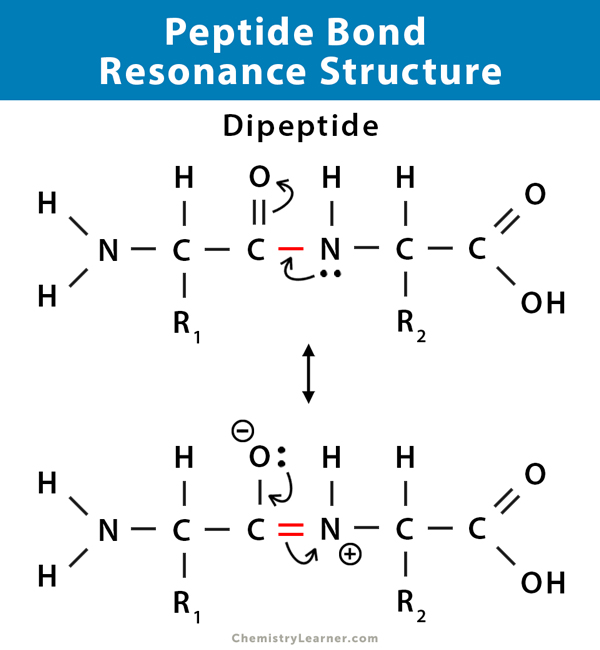
Peptide Bond Resonance Structure
The general structure of the peptide group is rigid and planar. The stability of a peptide bond is because of the resonance of the amide. A resonance structure forms due to the interaction between electrons of the carbonyl group’s double bond with those of the C–N bond. This effect is an example of resonance, which can be considered to share electrons between bonds. As a result, the C-N acquires a partial double bond property. The C=O bond acquires a partial single bond property.
Since single bonds between two atoms are longer than double bonds between the same two atoms, the lengths of the C–N and C=O bonds in a peptide differ from those observed in structures without resonance. Thus, the partial double bond character of C–N implies that this bond is shorter than would be predicted for a regular C–N single bond. On the other hand, the C=O bond is more extended than predicted for a regular C=O double bond [6].
Peptide Bond in Protein
A peptide consists of a chain of many amino acids. On the contrary, a protein contains a long chain of peptides with 50 or more amino acids. Therefore, proteins are long chains of amino acids held together by peptide bonds. Not all peptides form proteins, but all proteins consist of peptides. Typically, proteins display a more complex structure than simpler peptides.
The presence of proteins in a substance can be detected using a test called the Biuret test.
Peptide Bond Examples
A simple example of a peptide is the dipeptide called glycylglycine. It is formed from glycine residues.
Peptide Bond Hydrolysis
Peptide bond hydrolysis is the primary step of all reactions involving protein hydrolysis. The hydrolysis process occurs in the presence of water and is catalyzed by the presence of acid. It is essential in some synthetic reactions where amino acids in one peptide are cleaved and transferred to another peptide. This process results in separate peptide synthesis. Peptide bond hydrolysis is also an essential step in the digestion of proteins in living beings [1-5].
Importance of Peptide Bond
The peptide bond is one of the essential biochemistry reactions since it is used by amino acids to form proteins. The function of a peptide bond is to maintain the stability of proteins. In living organisms, proteins are used in many roles. These include physical support, catalyzing energetic reactions, and identifying molecules in the environment. The peptide bond is essentially the basis of many biological reactions. The formation of peptide bonds is a requirement for all life. This forming is very similar in all forms of life.
FAQs
Ans. During the formation of peptide bonds, an enzyme called peptidyl transferase catalyzes the addition of amino acid residues to grow the polypeptide chain.
Ans. A peptide bond is a type of amide bond. However, peptide bonds are present in long molecular chains, whereas amide bonds are observed in simple short molecules.
Ans. A peptide bond is formed between two amino acid molecules. Polypeptides are a continuous and long chain of peptide bonds with more than fifty monomer units of amino acids.
Ans. An example of a polysaccharide containing peptide bonds is peptidoglycan.