Covalent Bond
What is a Covalent Bond?
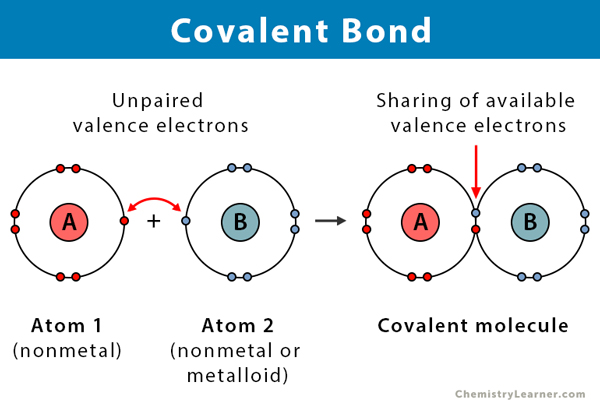
A covalent bond is a type of chemical bond that involves the sharing of electron pairs between atoms. The binding arises from the electrostatic attraction of their nuclei for the electrons. It is responsible for holding the atoms together. Atoms share electrons so that they can obtain a stable electronic configuration following the octet rule. Compounds that exhibit covalent bonds are known as covalent compounds. Covalent bonds are more common in organic chemistry than ionic bonds. They are also known as molecular bonds [1-9].
How are Covalent Bonds Formed
A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. Electronegativity is the ability of an atom to draw electrons to itself. Atoms will covalently bond with other atoms to gain more stability, obtained by sharing the outermost (valence) electrons and forming a complete electron shell.
Covalent bonds hold atoms together because the attraction between the positively charged nuclei and the negatively charged shared electrons is greater than the repulsions between the nuclei themselves. This attraction makes the molecules stable. The strength of a covalent bond is determined by the energy required to break it, that is, the energy necessary to separate the bonded atoms.
Properties and Characteristics of Covalent Bond
Covalent bonds are responsible for the general behavior of stable covalent compounds. Here are the properties and characteristics of a covalent bond [9]:
- Formed by the sharing of electrons between atoms
- Formed between two nonmetals or between a nonmetal and a metalloid
- There can be multiple covalent bonds between two atoms
- Bond is strong, and much energy is needed to break them
- Directional. A single molecular formula may represent several compounds with different properties – a phenomenon known as isomerism.
- Covalent compounds have low melting and boiling points
- Most covalent compounds do not conduct electricity
- Covalent compounds are insoluble in polar solvents like water. However, they dissolve in nonpolar solvents like benzene and toluene.
- Reactions of covalent compounds are relatively slow
Types of Covalent Bond
A covalent bond can be classified by the number of shared electrons, the polarity of bonds, and the coordination of the atoms.
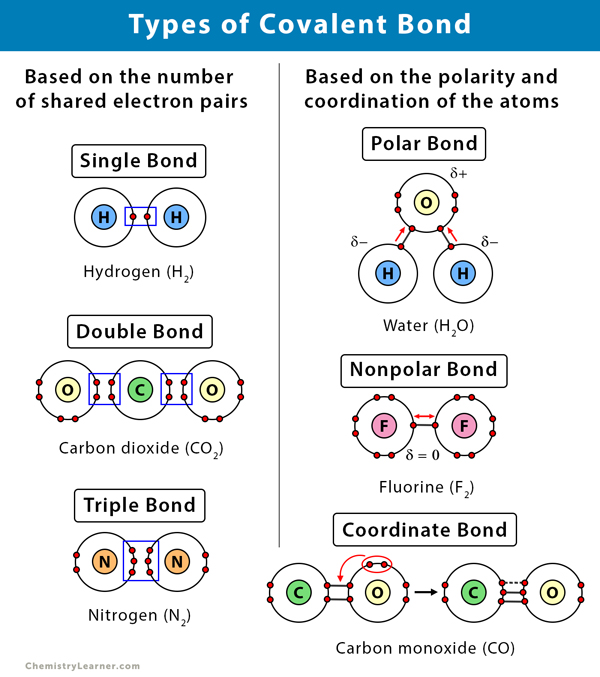
Based on the number of shared electron pairs, there are three types of covalent bonds [1-6]:
1. Single Covalent Bond
When one pair of electrons, or two electrons, are shared between the atoms, it is known as a single covalent bond or merely a single bond.
Examples: H2, Cl2, Br2, I2, HCl, NH3, CH4, and C2H6
2. Double Covalent bond
When two pairs of electrons, or four electrons, are shared between the atoms, it is known as a double covalent bond or double bond.
Examples: O2, CO2, SO2, and C2H4
3. Triple Covalent Bond
When three pairs of electrons, or six electrons, are shared between the atoms, it is known as a triple covalent bond or triple bond.
Examples: N2, C2H2, and CN–
Based on the polarity of the bond and the coordination of the atoms, there can be three other types of covalent bonds:
1. Polar Covalent Bond
A covalent bond is likely to be polar when the atoms sharing the electrons have a significant difference in their electronegativities, i.e., between 0.1 to 2. As a result, the bonded pair is attracted toward the more electronegative atom making that atom slightly negative, and the other atom becomes slightly positive.
Examples: H2O, CHCl3, CH3OH, HCl, and NH3
2. Nonpolar Covalent Bond
When the electronegativity difference between the atoms is zero, then electrons are equally shared between the atoms. In this case, the covalent bond is nonpolar.
Examples: H2, O2, N2, CO2, and CH4
3. Coordinate Covalent Bond or Dative Covalent Bond
In this type of covalent bond, the shared pair of electrons comes from one of the atoms. This kind of bond is typically observed in the bonding of metal ions to ligands.
Examples: BF3.NH3, Al2Cl6, HNO3, CO, H3O+, and NH4+
Examples of Covalent Bonds
Nonmetals like carbon, hydrogen, oxygen, and nitrogen form covalent bonds with themselves or other atoms. The number of covalent bonds that they can form is as follows:
Hydrogen – 1
Oxygen – 2
Nitrogen – 3
Carbon – 4
Here are some examples of covalent compounds [1-9]:
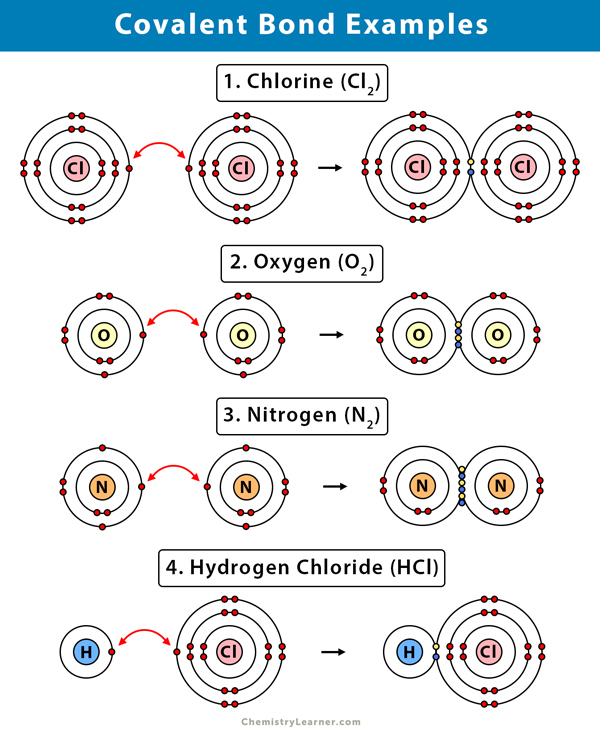
1. Hydrogen (H2)
Hydrogen (H) is the simplest of all elements. It has only one electron and requires another electron to achieve the electronic configuration of its nearest inert gas helium. So, two hydrogen atoms will bond together in a single bond to form a hydrogen molecule.
2. Oxygen (O2)
The valency of oxygen (O) is two, which means that it requires two electrons to complete its outermost (valence) shell. Therefore, two oxygen atoms will combine and share their two valence electrons, resulting in a double bond.
2. Nitrogen (N2)
Nitrogen (N) has five valence electrons, so it needs three more valence electrons to complete its octet. Two nitrogen atoms will combine. Each will share three electrons to form three covalent bonds, i.e., a triple bond, resulting in a nitrogen molecule.
3. Water (H2O)
A water molecule consists of two hydrogen (H) and one oxygen (O) atom. Oxygen has a valency of two, and hydrogen has only one electron in its orbital. So, each hydrogen atom will share its electron and covalently bond with the oxygen. As a result, there will be two single bonds.
4. Carbon Dioxide (CO2)
Carbon dioxide has two oxygen (O) atoms that are bonded to a single carbon (C) atom. The valency of carbon is four, and that of oxygen is two. So, each oxygen forms a double bond by sharing two of its valence electrons with the carbon. Hence, each C=O bond is a double bond.
5. Methane (CH4)
Methane is made up of one carbon (C) and four hydrogen (H) atoms. The valency of carbon is four, and that of hydrogen is one. So, each hydrogen will share its only electron and form a single covalent bond with the carbon. There will be a total of four covalent bonds in methane, all of which are single bonds.
6. Ammonia (NH3)
Nitrogen (N) has five electrons in its outer orbital and requires three more to complete its valence shell. Hydrogen (H) will share its lone electron with nitrogen, and three hydrogen atoms are required to complete nitrogen’s outermost shell. This sharing of electrons results in three single covalent bonds.
7. Carbon Monoxide (CO)
The carbon monoxide molecule is represented by three covalent bonds between the carbon (C) and oxygen (O) atoms. Carbon has a valency of four and will require four electrons to complete its outermost shell. Oxygen has a valency of two and requires two electrons to complete its outermost shell. Therefore, a regular double bond will form between the two atoms. Carbon is left with a deficit of two electrons, which will come from oxygen as it already has lone pairs. As a result, the third covalent bond will be a coordinate covalent bond.
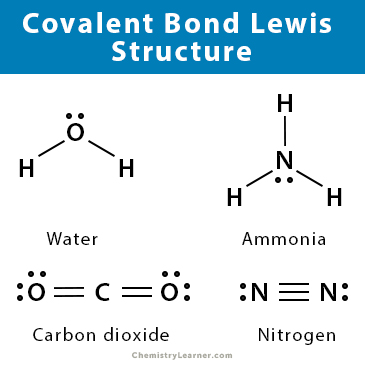
Covalent Bond Lewis Structure
Lewis dot structure can represent a molecule with covalent bonds. The valence electrons are shown as dots around the atom, and a dashed line shows the bond with a neighboring atom.
Pure Covalent Bonds
By definition, a pure covalent bond is one that exists between two atoms with the same electronegativities. Thus, a pure covalent bond does not display any ionic character. Diatomic elements are perfect examples of pure covalent bonds because both atoms evenly share the electrons.
Examples: H2, O2, and N2
FAQs
Ans. No. Hydrogen bonds are not covalent bonds.
Ans. No. NaCl is not a covalent bond. It is an ionic bond.
Ans. No. Covalent bonds give water a high heat capacity.
Ans. Yes. A peptide bond is a type of covalent bond.
Ans. Ozone has covalent bonds. The reason is that in ozone, the atoms are associated by sharing electrons within them.