Photochemical Reaction
- What is a Photochemical Reaction? [1-5]
- Importance of Photochemical Reaction [3]
- Basic Principle of Photochemical Reaction [4-6]
- Types of Photochemical Reaction [7]
- Examples of Photochemical Reaction [2-5]
- Photochemical Reaction in Atmosphere [8-10]
- Photochemical Smog Reactions [8-10]
- Applications of Photochemical Reaction
- Difference Between Photochemical Reaction and Thermal Reaction
- Difference Between Photochemical Reaction and Electrochemical Reaction
- FAQ
What is a Photochemical Reaction? [1-5]
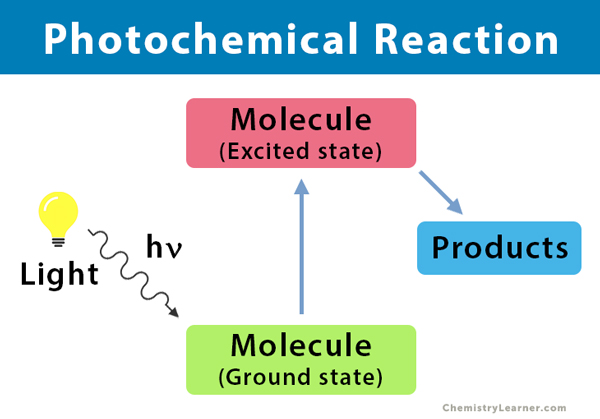
Photochemistry is the branch of chemistry that deals with the chemical processes that are caused by the absorption of light energy. A photochemical reaction is a chemical reaction initiated by the absorption of energy in the form of light (photons), resulting in specific products. Usually, molecules prefer to remain in the state of lowest energy, known as the ground state. When they are excited by photons, molecules absorb energy and goes into a transient state, known as the excited state. In this state, the physical and chemical properties of the molecules are entirely different from that of the ground state.
Photochemical reactions are driven by the number of photons that can activate molecules to cause the desired reaction. During a photochemical reaction, these molecules tend to form a new structure. They could combine with each other or with other molecules and transfer electrons, atoms, protons, or other excitation energy to other molecules, thus causing a prolonged chemical chain reaction. The photochemical reaction can take place in solid, liquid, and gas.
Historical developments in photochemistry took place in the early 1800s. In 1817, German physicist Theodor von Grotthus developed a theoretical understanding of the photochemical process. Later, in 1841, American chemist John William Draper studied the photochemical reaction between hydrogen and chlorine gases.
Importance of Photochemical Reaction [3]
Photochemical reactions are of great importance for the support of life on Earth. Chemical changes taking place in the atmospheric gases of the Earth are initiated by solar radiation and modified by the suspended particles. The study of upper atmospheric photochemical reactions has significantly contributed to the knowledge of depletion of the ozone layer, acid rain, and global warming.
Photochemical reactions have a particular advantage over other types of reactions. Photochemical reactions require sunlight, which is abundantly available. With the sun as its central figure, the origin of life itself must have been a photochemical process in the primitive earth conditions as radiation from the sun was the only source of energy. Simple gaseous molecules like methane, ammonia, and carbon dioxide must have reacted photochemically to synthesize complex organic molecules like proteins and nucleic acid through the ages.
The photochemical process demonstrates perfect atom economy, as the transformation is initiated by a photon, rather than an extra reagent.
Basic Principle of Photochemical Reaction [4-6]
A photochemical reaction is based on the principles of photochemistry. When light shines on a molecule, it goes to an excited state, a process known as photoexcitation. There are two laws of photochemical reaction:
- Grothuss-Draper Law: This law states that a molecule must absorb light in order for a chemical reaction to take place.
- Stark-Einstein Law: This law states that for each photon of light absorbed by a molecule, only one molecule is activated for a subsequent reaction.
The efficiency with which a given photochemical process occurs is given by a term called quantum yield. Quantum yield is defined as “the number of moles of a stated reactant disappearing, or the number of moles of a stated product produced, per mole of a photon of monochromatic light absorbed.” Since many photochemical reactions are complex and may compete with unproductive energy loss, the quantum yield is usually specified for a particular event.
Types of Photochemical Reaction [7]
Here are the types of photochemical reactions:
- Photo-dissociation: AB + hν → A* + B*
- Photo-induced rearrangements, isomerization: A + hν → B
- Photo-addition: A + B + hν → AB
- Photo-substitution: A + BC + hν → AB + C
- Photo-redox reactions: A + B + hν → A– + B+
Examples of Photochemical Reaction [2-5]
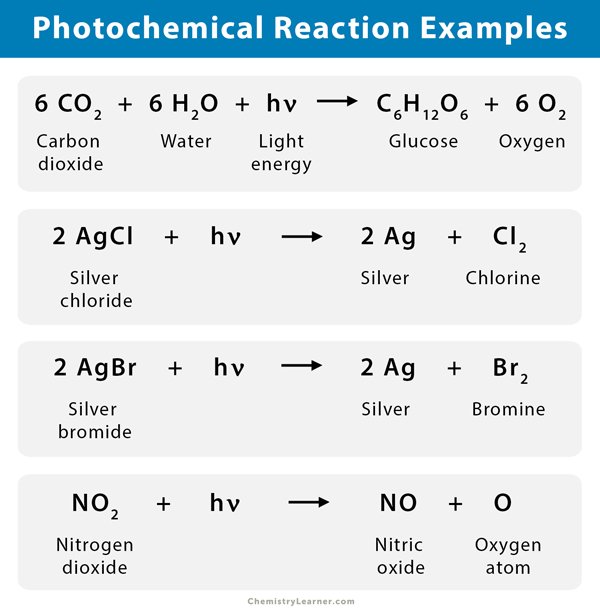
- During a photosynthesis process, the pigment chlorophyll in plants takes in the energy (hν) from the sun and water (H2O) to convert carbon dioxide (CO2) into glucose (C6H12O6) and oxygen (O2). Photosynthesis can also be carried in the presence of artificial light.
6 CO2 + 6 H2O + hν → C6H12O6 + 6 O2
- Photography uses the action of light on grains of silver chloride (AgCl) or silver bromide (AgBr) to produce an image. Silver halides (AgX) decomposes into silver (Ag) and halogen (X2). This reaction is an example of a photochemical decomposition reaction.
2 AgCl + hν → 2 Ag + Cl2
2 AgBr + hν → 2 Ag + Br2
- Solar cells, which are used to power satellites and space vehicles, convert light energy from the sun to chemical energy and then release that energy in the form of electrical energy.
- Formation of vitamin D by exposure of skin to sunlight
- Carbonyl compounds undergo various photochemical reactions in both gas and liquid phases
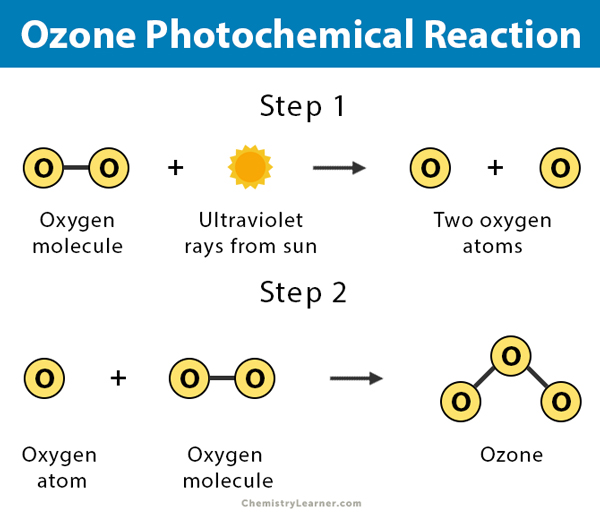
- Ozone formation in the upper atmosphere results from the action of sunlight on oxygen molecules.
3 O2 + hν → 2 O3
Photochemical Reaction in Atmosphere [8-10]
The atmosphere contains some gaseous substances which locally alters the chemical composition of air. From the kinetic molecular theory of gases, the molecules present in the atmosphere are moving and colliding together continuously. In the day time, solar radiations are continuously delivered to the atmosphere. As a result, the molecules present in the atmosphere absorb the light energy, and photochemical reactions occur. Photochemical reactions play a crucial role in determining the nature of chemical species, including pollutant species, in the atmosphere. The oxidation reactions taking place in the atmosphere are driven by solar energy.
Photochemical Smog Reactions [8-10]
Photochemical smog is a mixture of pollutants that are formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react to sunlight, creating a brown haze above cities. It is a byproduct of modern industrialization. Photochemical smog can affect the environment, people’s health, and even various materials. Chemicals such as nitrogen oxides, ozone, and peroxyacetyl nitrate (PAN) can have harmful effects on plants.
NOx and VOCs are the primary pollutants, whereas ozone, aldehydes, and PAN are secondary pollutants. Ozone in the atmosphere protects us from the ultraviolet rays of the sun, but, at the ground level, it is quite dangerous. Here are the reactions that take place in the atmosphere, ultimately leading to smog:
1. Nitrogen dioxide (NO2) absorbs ultraviolet light, and the formation of nitric oxide (NO) and atomic oxygen (O) takes place.
NO2 + hν → NO + O
2. Ozone (O3) is generated by the reaction of oxygen (O2) gas with this atomic oxygen.
O2 + O → O3
3. Ozone so formed then reacts with NO to form NO2 and O2:
NO + O3 → NO2 + O2
4. PAN is produced by reactions of nitrogen dioxide with various hydrocarbons (RH), coming from VOCs:
NO2 + RH → PAN
5. Oxygenated organic and inorganic compounds (ROx) react with nitric oxide to produce more nitrogen oxides:
NO + ROx → NO2 + other products
Applications of Photochemical Reaction
Here are some industrial applications of photochemical reactions:
- For the preparation of anti-malaria drug
- For the production of benzyl chloride
- For the production of various synthetic organic molecules
Difference Between Photochemical Reaction and Thermal Reaction
Photochemical Reaction vs. Thermal Reaction |
||
| Photochemical Reaction | Thermal Reaction | |
|---|---|---|
Definition | Takes place due to the absorption of radiations (photons) by molecules | Takes place due to the absorption of heat energy, generally by an increase of the temperature of the reaction medium |
Source | Light | Heat |
Effect of light | Appropriate light source is necessary | Reaction can occur in the absence of light |
| Effect of temperature | Temperature has no effect | Temperature has a direct effect |
Acceleration | Catalyst is not required to accelerate the reaction. However, a high intensity of light can increase the rate of reaction. | Most reactions require a catalyst to accelerate the reaction |
Difference Between Photochemical Reaction and Electrochemical Reaction
Photochemical Reaction vs. Electrochemical Reaction |
||
| Photochemical Reaction | Electrochemical Reaction | |
|---|---|---|
Definition | Takes place due to the absorption of radiations (photons) by molecules | Takes place due to the passage of electric current |
Source | Light | Electricity |
Example | Photosynthesis | Reactions in an electrical cell |
FAQ
Ans. The reverse of the photochemical reaction is called chemiluminescence.