Double-replacement Reaction
- What is a Double-replacement Reaction?
- Why does Double-replacement Reaction Occur?
- What Happens in a Double-replacement Reaction?
- How to Identify a Double-replacement Reaction?
- Examples of Double-replacement Reaction
- How to Balance a Double-replacement Reaction?
- Double-replacement Reaction Rules
- Types of Double-replacement Reaction
- Double-replacement Reaction Examples in Real Life
- FAQs
What is a Double-replacement Reaction?
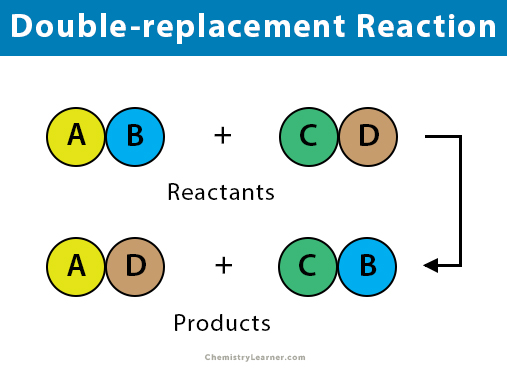
A double-replacement reaction, also known as a double displacement reaction, is a type of chemical reaction in which two reactants exchange ions to form two new compounds. Double-replacement reaction takes place in an aqueous solution and typically results in the formation of a precipitate. Ionic compounds mostly participate in a double-replacement reaction, so do acids and bases. The bonds formed in the product compounds are the same type of bonds, as seen in the reactant molecules [1-3].
General Equation
The general formula of double-replacement reaction is:
AB + CD → AD + BC
When writing an actual reaction, the reaction must be balanced. It is often useful to denote an aqueous solution as aq. and a precipitate as s/ppt.
Why does Double-replacement Reaction Occur?
A double-replacement reaction occurs when two ionic compounds react. The positive ions (cation) and negative ions (anion) of the two reactants, which are ionic compounds, exchange their places. The reaction results in two new products, which are also ionic compounds.
What Happens in a Double-replacement Reaction?
A double-replacement reaction exchanges the cations or anions of two ionic compounds. The two compounds are in their aqueous solutions react to form an insoluble precipitate, an insoluble gas, or water.
How to Identify a Double-replacement Reaction?
The easiest way to identify a double displacement reaction is to check to see whether or not the cations exchanged anions with each other. Another clue, if the states of matter are cited, is to look for aqueous reactants and the formation of one solid product since the reaction typically generates a precipitate.
Examples of Double-replacement Reaction
An example of a double-displacement reaction is the reaction between iron (III) bromide (FeBr3) and sodium hydroxide (NaOH). The products are sodium bromide (NaBr) and iron (III) hydroxide (Fe(OH)3) precipitate.
FeBr3 (aq) + 3 NaOH (aq) → Fe(OH)3 (s/ppt.) + 3 NaBr (aq.)
How to Balance a Double-replacement Reaction?
Consider the reaction between ammonium sulfate ((NH4)2SO4) and barium nitrate (Ba(NO3)2). The products are barium sulfate (BaSO4) and ammonium nitrate (NH4NO3).
(NH4)2SO4 (aq) + Ba(NO3)2 (aq) → NH4NO3 (aq) + BaSO4 (s/ppt.)
Here, the above reaction is unbalanced. We note that the anions SO42- and NO3– interchange their places to form the products. However, we find that there are only one NH4– and one NO3– on the right-hand side and two of each ion on the left. So, we multiply the compound NH4NO3 by 2 to balance the equation.
(NH4)2SO4 (aq) + Ba(NO3)2 (aq) → 2 NH4NO3 (aq) + BaSO4 (s/ppt.)
Double-replacement Reaction Rules
How to Predict a Double-replacement Reaction?
The fundamental rule that a double-replacement reaction follows is the solubility rule. This rule is used to predict whether a precipitate will form or not. The formation of a solid precipitate is the driving force that makes the reaction proceed in the forward direction. The following table of solubility can be used to predict the formation of a precipitate [3].
| Compounds soluble in water | Exception |
| All compounds of Li+, Na+, K+, Rb+, Cs+, and NH4+ | None |
| All compounds of NO3− and C2H3O2− | None |
| Compounds of Cl−, Br−, and I− | Ag+, Hg22+, and Pb2+ |
| Compounds of SO42- | Hg22+, Pb2+, Sr2+, and Ba2+ |
| Compounds insoluble in water | Exception |
| Compounds of CO32− and PO43− | Compounds of Li+, Na+, K+, Rb+, Cs+, and NH4+ |
| Compounds of OH− | Compounds of Li+, Na+, K+, Rb+, Cs+, NH4+, Sr2+, and Ba2+ |
Types of Double-replacement Reaction
There are two types of double-replacement reactions [1-5].
1. Precipitation Reaction
A precipitation reaction occurs when two ionic compounds are dissolved in water and form a new ionic compound that does not dissolve. This new compound settles down in the solution as a solid precipitate. The formation of a solid precipitate is the driving force that makes the reaction proceed.
Examples
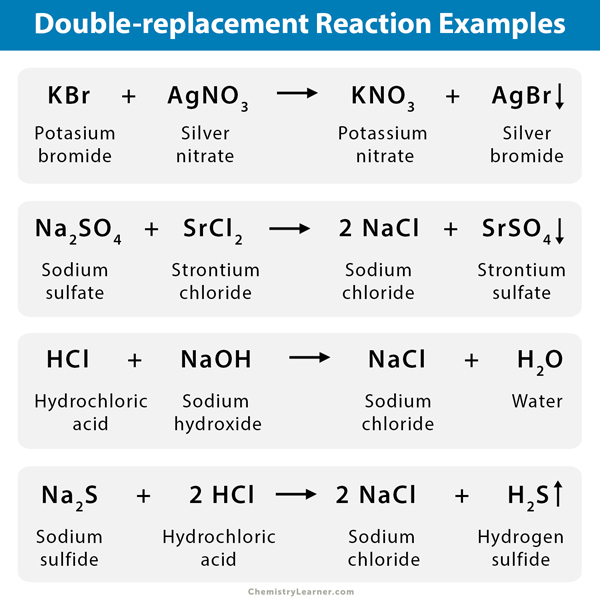
- The reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) results in silver chloride (AgCl) precipitate in sodium nitrate solution (NaNO3).
NaCl (aq.) + AgNO3 (aq.) → NaNO3 (aq.) + AgCl (s/ppt.)
- The reaction between barium chloride (BaCl2) and sodium sulfate (Na2SO4) results in sodium chloride (NaCl) and a white precipitate of barium sulfate (BaSO4).
BaCl2 (aq.) + Na2SO4 (aq.) → NaCl (aq.) + BaSO4 (s/ppt.)
- The reaction between lead (II) nitrate (Pb(NO3)2) and potassium iodide (KI) leads to the formation of insoluble lead (II) iodide (PbI2) in potassium nitrate (KNO3) solution.
2 KI (aq.) + Pb(NO3)2 (aq.) → 2 KNO3 (aq.) + PbI2 (s/ppt.)
2. Acid-Base Reaction or Neutralization Reaction
The reaction between an acid and a base is called an acid-base reaction or a neutralization reaction. An aqueous acid-base reaction generally produces water and a new ionic compound, called a salt. It is preferable to use strong acid and a strong base to ensure that the reaction proceeds in the forward direction.
Examples
- A mixture of sulfuric acid (H2SO4) and sodium hydroxide (NaOH) produces sodium sulfate (Na2SO4) and water (H2O).
H2SO4 (aq.) + 2 NaOH (aq.) → Na2SO4 (aq.) + 2 H2O (l)
- A mixture of hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H2O).
HCl (aq.) + NaOH (aq.) → NaCl (aq.) + H2O (l)
- The reaction between vinegar (acetic acid) (CH3COOH) and baking soda (sodium bicarbonate) (NaHCO3) produces carbonic acid (H2CO3) and sodium acetate (CH3COONa).
NaHCO3 (aq.) + CH3COOH (aq.) → H2CO3 (aq.) + CH3COONa (aq.)
The carbonic acid is unstable. It undergoes decomposition reaction and releases carbon dioxide (CO2), which is responsible for the fizz caused by the reaction.
H2CO3 (aq.) → CO2 (g) + H2O (l)
Another Type of Double-replacement Reaction
Gas Formation
A gas formation reaction is one which yields a gas as a product. The gaseous product then bubbles out of the solution and escapes into the air.
Example
- When solutions of sodium sulfide (Na2S) and hydrochloric acid (HCl) are mixed, the products of the reaction are aqueous sodium chloride (NaCl) and hydrogen sulfide (H2S) gas.
Na2S (aq.) + 2 HCl (aq.) → 2 NaCl (aq.) + H2S (g)
- When zinc sulfide (ZnS) is dissolved in hydrochloric acid (HCl), the products are aqueous zinc chloride (ZnCl2) and hydrogen sulfide (H2S) gas.
ZnS (s) + 2 HCl (aq.) → ZnCl2 (aq.) + H2S (g)
Double-replacement Reaction Examples in Real Life
The double-replacement reaction can be observed in nature and real life.
The reaction between vinegar and baking soda has a few applications in daily life.
- As a cleaning agent, especially to clean the water disposal pipes in the kitchen and bathroom sinks.
- Can be collected and used as a chemical fire extinguisher
- To make a chemical volcano.
Some other examples are:
- Hard water reacts with soaps resulting in soap scum on the sink or the wall of a bathroom
- Hard water also causes deposition of minerals like calcium carbonate on the interior walls of water pipes
FAQs
Ans. No. For it to be a redox reaction, elements have to change oxidation states, and that does not happen in a double displacement reaction.
Ans. In a single-replacement reaction, a more reactive element replaces a less reactive element from a compound. In a double-replacement reaction, two atoms or a group of atoms switch places to form new compounds.