Barfoed’s Test
Definition: What is Barfoed’s Test?
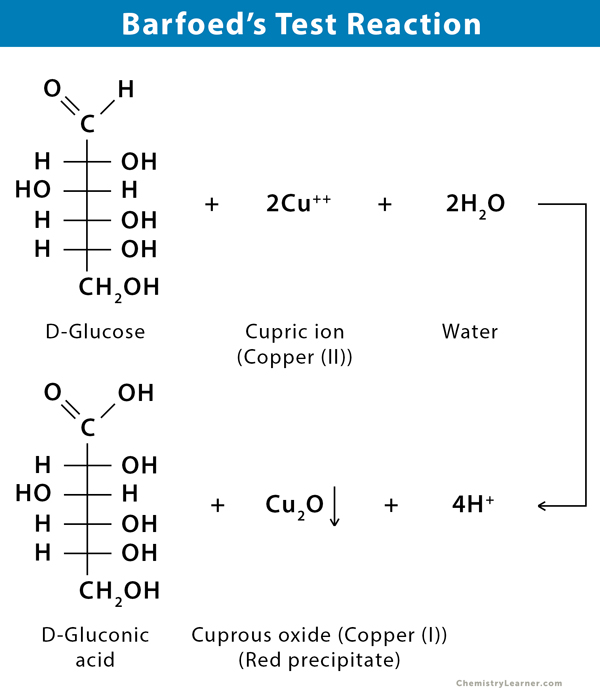
Barfoed’s test is a chemical test used for detecting the presence of monosaccharides. It is based on the reduction of cupric (II) acetate to cuprous (I) oxide (Cu2O), which forms a brick-red precipitate. Disaccharides may react, but the reaction is much slower because they have to get hydrolyzed first and then react with the reagent cupric acetate to produce cuprous oxide.
This test is named after the Danish chemist Christen Thomsen Barfoed, who reported it in a journal in 1873.
Barfoed’s Test Principle
Aldoses and ketoses can reduce cupric ions, even in acidic conditions. This test is used to distinguish reducing monosaccharides from disaccharides by controlling pH and the time of heating.
Barfoed’s Reagent Preparation
Barfoed’s reagent is the reagent used in this test. It is prepared by adding a 0.33 molar solution of neutral cupric (II) acetate to a 1% acetic acid solution.
Barfoed’s Test Procedure
- One ml of a sample solution is placed in a test tube.
- Three ml of Barfoed’s reagent is added.
- The solution is then heated in a boiling water bath for two minutes and allowed to cool.
- Record color and record the time required to develop a red precipitate.
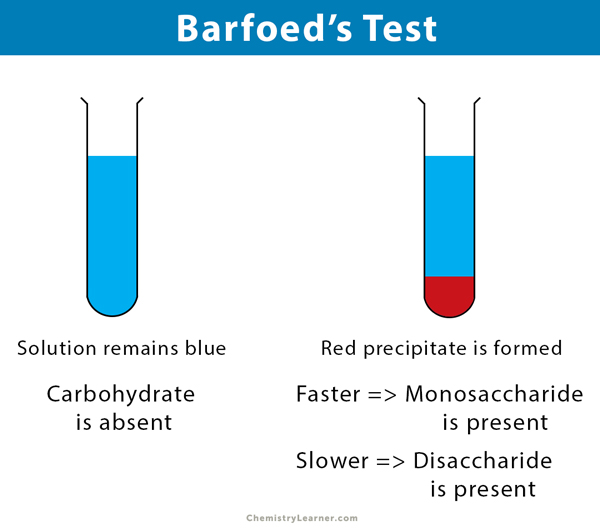
Barfoed’s Test Results
If a red precipitate is formed within two minutes, it means that a monosaccharide is present. If the red precipitate is formed after ten minutes of heating, a disaccharide is present.
Difference between Barfoed’s Test and Benedict’s Test
- Barfoed’s reagent is similar to Benedict’s reagent except that the pH is lower (around 4.5), and the heating time is reduced to two minutes.
- Benedict’s test would determine if the sample is a reducing sugar, and Barfoed’s test would determine if it is a monosaccharide or disaccharide.





I would like to be receiving an update