Hofmann Rearrangement
Definition: What is Hofmann Rearrangement?
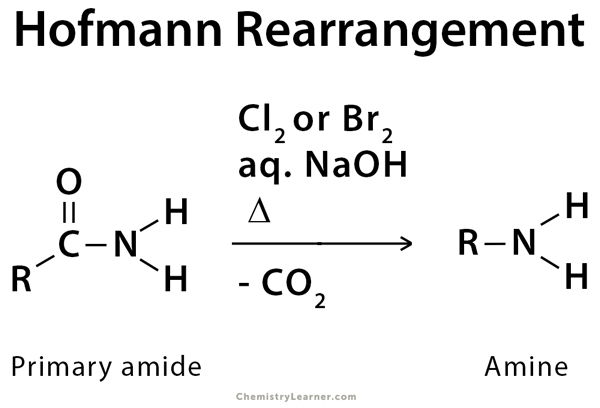
Hofmann rearrangement, also known as Hofmann degradation, is the reaction of a primary amide with a halogen (chlorine or bromine) in a strongly basic (sodium or potassium hydroxide) aqueous medium to convert the amide into a primary amine. The reaction results in one carbon degradation [1-5].
The history of this reaction goes back to 1881 when German chemist August Wilhelm von Hofmann reported it in a journal paper.
Example of Hofmann Rearrangement
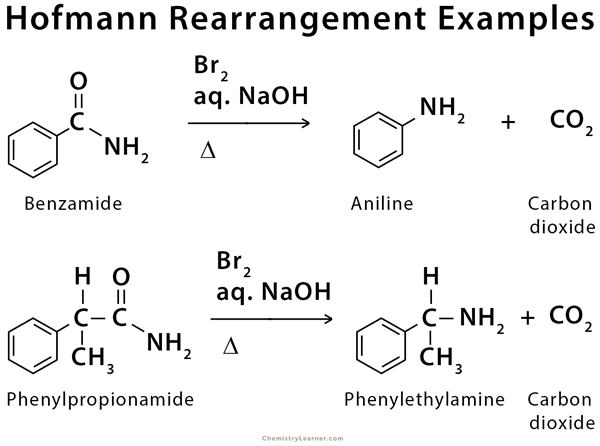
Benzamide (a primary amide) reacts with bromine (Br2) and aqueous sodium hydroxide (NaOH) to produce aniline (a primary amine) and carbon dioxide (CO2) [1-2].
Mechanism of Hofmann Rearrangement
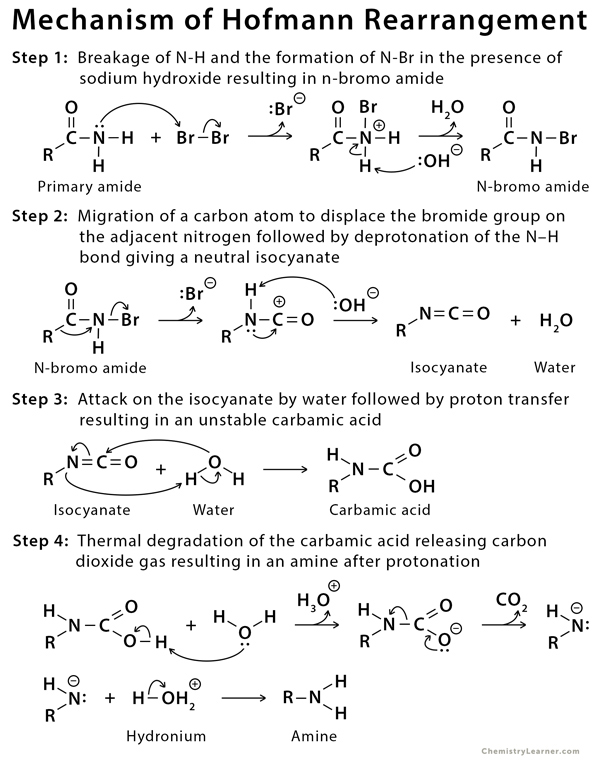
An amide is treated with bromine and aqueous sodium hydroxide (NaOH). Upon heating, an intermediate isocyanate is formed, which is not isolated. When water is added, the isocyanate loses carbon dioxide and forms the amine [3-5].
Application of Hofmann Rearrangement
Hofmann rearrangement can be used to prepare anthranilic acid from phthalimide. Anthranilic acid has a wide range of industrial applications including the preparation of perfumes, azo dyes, and saccharin – an artificial sweetener. This rearrangement reaction is also used to convert nicotinic acid into 3-aminopyridine [6,7].
References
- Definition, example, and mechanism – Webstor.srmist.edu.in
- Definition and example – Chem.ucla.edu
- Definition and mechanism – Chem.libretexts.org
- Definition and mechanism – Www.name-reaction.com
- Definition and mechanism – Ochempal.org
- Applications – Slideshare.net
- Applications – Slideshare.net