Claisen Condensation
Definition: What is Claisen Condensation?
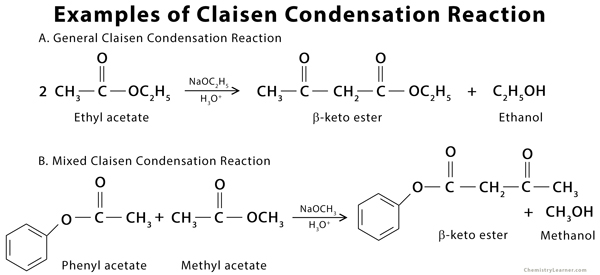
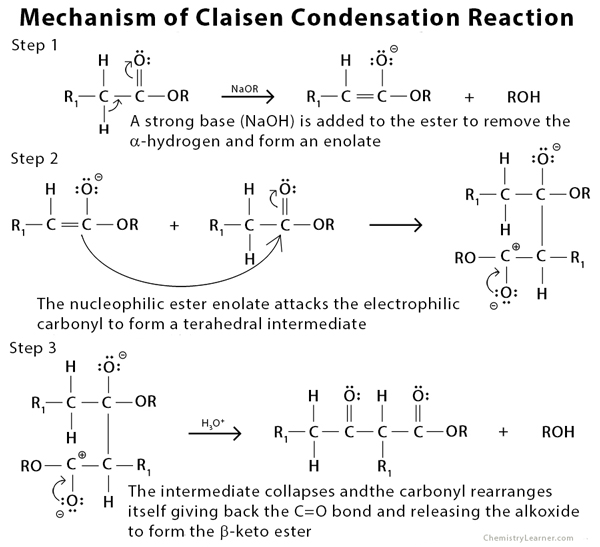
The Claisen condensation is an organic reaction used to form a carbon-carbon bond between two ester molecules or one ester molecule with another carbonyl compound in the presence of a strong base (alkoxide). The product formed is a beta-keto ester. During the reaction, an enolate is formed when the alpha-hydrogen is removed from the ester. If the ester lacks an alpha-hydrogen, as with most aromatic carbonyl compounds, it is incapable of forming the enolate. Such a type of condensation reaction is called crossed or mixed Claisen condensation [1 -3].
Examples of Claisen Condensation Reaction
Mechanism of Claisen Condensation Reaction [4 – 7]
Claisen-Schmidt Condensation
In the case of Claisen-Schmidt Condensation, an aldehyde or a ketone having an alpha-hydrogen is used to react with an aromatic carbonyl compound without any alpha-hydrogen.
FAQs
Q.1. Why cannot methanoate esters undergo Claisen self-condensations?
Ans. Methanoate esters (HCOOR) don’t have an alpha-hydrogen and therefore, cannot form the nucleophilic enolate.
Q.2.What type of esters can undergo Claisen condensation reactions?
Ans. Only those esters that contain alpha-hydrogen can undergo Claisen Condensation reaction.
Q.3. What is a beta-keto ester?
Ans. A beta-keto ester is a compound that is formed by joining a ketone and an ester with carbon between them.
- References
- Definition and mechanism – Name-reaction.com
- Definition – Chem.ucla.edu
- Definition – Chem.ucalgary.ca
- Mechanism – Chemhelper.com
- Mechanism – Chem.libretexts.org
- Mechanism – Organic-chemistry.org
- Mechanism – Clutchprep.com