Lanthanides
Definition: What are lanthanides?
Lanthanides belong to the category of inner transition metals with a partially filled 4f shell. They are called lanthanides because they have chemical properties similar to lanthanum, the first element in the series [1].
Where are the lanthanides located in the periodic table?
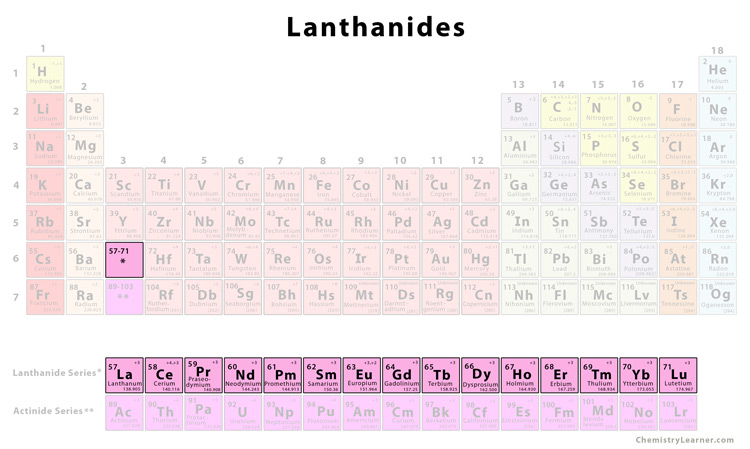
The lanthanides are located in group 3 of the 6th period of the periodic table in between barium (Ba) and hafnium (Hf). They are shown below the body of the periodic table for simplicity. They are also known as f-block elements since the 4f shell progressively fills as the atomic number changes from 57 to 71. The lanthanides have a general electronic configuration given by [Xe] 4f1-145d0-16s2, where [Xe] is the electronic configuration of the nearest noble gas xenon [1, 2].
List of lanthanides [2]There are a total of 15 lanthanides in the periodic table. |
||
| Element | Symbol | Atomic Number |
| Lanthanum | La | 57 |
| Cerium | Ce | 58 |
| Praseodymium | Pr | 59 |
| Neodymium | Nd | 60 |
| Promethium | Pm | 61 |
| Samarium | Sm | 62 |
| Europium | Eu | 63 |
| Gadolinium | Gd | 64 |
| Terbium | Tb | 65 |
| Dysprosium | Dy | 66 |
| Holmium | Ho | 67 |
| Erbium | Er | 68 |
| Thulium | Tm | 69 |
| Ytterbium | Yb | 70 |
| Lutetium | Lu | 71 |
Common Properties and Characteristics of the Lanthanides
Lanthanides have similar properties to one another and display several physical, chemical, and magnetic features.
Physical Properties of Lanthanides [1]
- Soft and silvery-white metals which can be easily cut with a knife
- Hardness increases across the series
- Atomic radii successively decrease as we go from left to right of the periodic table (lanthanide contraction)
- Have high electrical resistivity
Chemical Properties of Lanthanides [1]
- Form colored solutions which turn pale yellow
- Display several oxidation states, with +3 being the most common
- They are very reactive metals and can quickly form oxide coatings upon exposure to air, except for gadolinium and lutetium, which slowly discolor in air
- Prefer highly electronegative elements for binding
- Quickly dissolve in acid, ignite in air, and reacts with halogens, sulfur, hydrogen, carbon, and nitrogen upon heating
Magnetic Properties of Lanthanides [3, 4]
- Some lanthanide ions are diamagnetic (e.g., La3+, Lu3+, Yb2+, Ce4+) because they do not have unpaired electrons, while the rest of the elements are paramagnetic at room temperature.
- Ferromagnetic below Curie temperature, which is generally very low (e.g., 16 °C for Gd)
Uses and Applications of Lanthanides [5]
- To impart strength and hardness to materials (e.g., cerium)
- In metallurgical applications, due to strong reducing capability (e.g., cerium)
- As a catalyst to produce glasses
- In cathode ray tubes of old television sets (e.g., lanthanum ions)
- As luminescent in Nd: YAG laser and in optical devices like night vision goggles (e.g., lanthanum ions)
- Possible medical use as an anticancer agent (e.g., cerium and lanthanum)
Occurrence of Lanthanides
Lanthanides belong to the category of rare earth elements. They are called rare earth because the process of separating pure metals from ores was complicated, and they were rare to obtain. The occurrence of rare earth elements in the earth’s crust is relatively abundant. Cerium is the most abundant, and thulium is the least abundant [6].
Minerals of Lanthanides
Lanthanides are obtained from several minerals that are available in the earth’s crust. The minerals that are mined include bastnasite, laterite clays, monazite, and loparite. Among these, bastnasite is the principal source of rare earth elements and is widely found in California, USA [6].
Lanthanide Contraction
Lanthanide contraction is a phenomenon in which the atomic radii of the lanthanides decrease as the atomic numbers increase across the series. This phenomenon is because as more and more electrons are filling, the 4f electrons cannot provide perfect shielding from the nucleus to the 6s electrons. As a result, the effective nuclear charge increases, and the 6s electrons come closer to the nucleus, decreasing the atomic radius [7].
FAQs
Q.1. Why are lanthanides called inner transition elements?
Ans. Lanthanides are called inner transitional elements because of their position in the periodic table. They are placed between the s-block element barium and the d-block element hafnium.
Q.2. What is an older name for lanthanides?
Ans. Lanthanides come from lanthanum, which used to be called a rare earth element at the time of discovery.
Q.3. Are lanthanides radioactive?
Ans. Promethium, whose all isotopes are radioactive, is the only lanthanide with such properties. The rest of the elements in the family are not radioactive.
Q.4. How reactive are lanthanides?
Ans. Some lanthanides, namely La, Ce, Pr, Nd, and Eu, are very reactive. When exposed to air, they react with oxygen and turn into a pale color.
References
- Definition of lanthanides – Thoughtco.com
- List of lanthanides – Coolperiodictable.com
- Properties of lanthanides – Chem.libretexts.org
- Magnetic properties of lanthanides – Radiochemistry.org
- Applications of lanthanides – Ck12.org
- Occurrence and minerals of lanthanides – Science
- Lanthanide contraction – Britannica.com