Transition Metals
Definition: What are transition metals?
Transition metals are a group of metals in the middle of the periodic table that have some similar properties to one another. A unique feature of these metals is that they have valence electrons in two shells, and electrons from both these shells participate in chemical bonding. As a result, they show several common oxidation states [1].
For inner transition metals, refer to lanthanide and actinide.
Where are transition metals located in the periodic table?
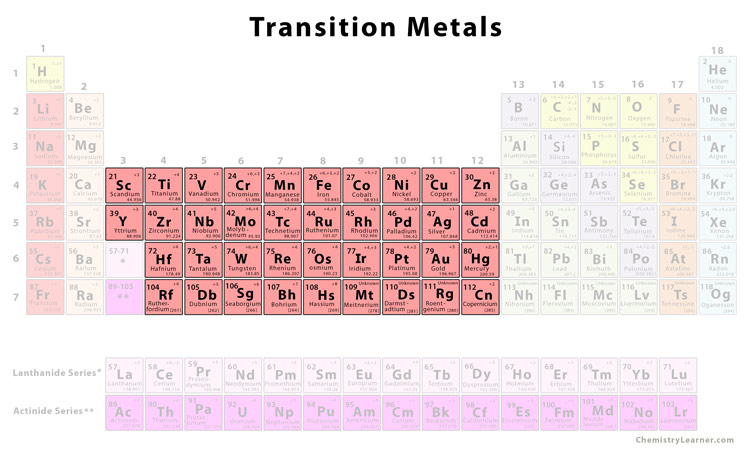
The transition metals are located in the middle of the periodic table in between the elements of the left and the right. They act as a bridge or transition between the two sides of the periodic table. They are also called d-block elements since they occupy the space between the s-block and the p-block elements of the periodic table and have their d orbitals filled progressively. They range from Group 3 (IIIb) to Group 12 (IIb) with a general configuration given by (n-1)d1-10ns1,2. Although in d-block, scandium and zinc are not considered transition metals because their stable ions do not have any d electrons to form complex compounds [2, 3, 4].
List of Transition Metals [5] |
||
| Element | Symbol | Atomic Number |
| Titanium | Ti | 22 |
| Vanadium | V | 23 |
| Chromium | Cr | 24 |
| Manganese | Mn | 25 |
| Iron | Fe | 26 |
| Cobalt | Co | 27 |
| Nickel | Ni | 28 |
| Copper | Cu | 29 |
| Yttrium | Yt | 39 |
| Zirconium | Zr | 40 |
| Niobium | Nb | 41 |
| Molybdenum | Mo | 42 |
| Technetium | Tc | 43 |
| Ruthenium | Ru | 44 |
| Rhodium | Rh | 45 |
| Palladium | Pd | 46 |
| Silver | Ag | 47 |
| Cadmium | Cd | 48 |
| Hafnium | Hf | 72 |
| Tantalum | Ta | 73 |
| Tungsten | W | 74 |
| Rhenium | Re | 75 |
| Osmium | Os | 76 |
| Iridium | Ir | 77 |
| Platinum | Pt | 78 |
| Gold | Au | 79 |
| Mercury | Hg | 80 |
| Rutherfordium | Rf | 104 |
| Dubnium | Db | 105 |
| Seaborgium | Sg | 106 |
| Bohrium | Bh | 107 |
| Hassium | Hs | 108 |
| Meitnerium | Mt | 109 |
| Darmstadtium | Ds | 110 |
| Roentgenium | Rg | 111 |
| Copernicium | Cn | 112 |
Common Properties and Characteristics of Transition Metals
The transition metals are very hard and heavy. They are classified into the 1st, 2nd, and 3rd transition series, with elements across the 1st series (Ti to Cu) showing similar physical and chemical properties. This is due to the small difference in the effective nuclear charge of these elements as a result of the shielding of the 4s electrons by the filling 3d electrons [6].
Physical Properties of Transition Metals [5, 6]
- Shiny and silvery
- High density
- High melting and boiling points
- Good conductors of heat and electricity, especially copper and silver
- Gold and silver are malleable, while copper is very ductile
Chemical Properties of Transition Metals [5, 6, 7]
- Less reactive than alkali metals
- Display several oxidation states [7]
- Have a tendency to form colored ions and compounds (e.g. CuSO4 is a blue crystal)
- Form stable complex compounds with other elements (e.g. halides such as FeCl2, NiCl2)
- Form positively charged molecular complexes with other atoms (e.g. CrO42-)
- Iron and titanium react with oxygen to form oxides
Magnetic Properties of Transition Metals [8]
- Iron, nickel, and cobalt show magnetic behavior up to a certain temperature
- Iron is ferromagnetic up to 770 °C (Curie point), beyond which it is paramagnetic, i.e. weakly attracted to a magnetic field
- Nickel loses its ferromagnetic properties at its Curie temperature of 355 °C
- Cobalt is ferromagnetic until it becomes paramagnetic at its Curie temperature of 1115 °C
Why are transition metals colored?
Colors in transition metals are due to electron transition, namely charge transfer transition and d-d transition. A charge transfer transition occurs when the metal is in a high or low oxidation state, during which charge is transferred between the metal and a nonmetal species (ligand). In the d-d transition, an electron moves from one d orbital to another. In complexes of transition metals, these d orbitals have different energies. When white radiation falls in these complex compounds, the d electron absorbs some radiation, and the rest is transmitted. Color is perceived as the radiation transmitted by these compounds [9].
List of colors of transition metal ions and ion complexes [10] |
|
| Transition metal ion | Color |
| Fe3+ | Brown to yellow |
| Co2+ | Pink |
| Cu2+ | Blue-green |
| Ni2+ | Bright green |
| Fe2+ | Olive green |
| Ti3+ | Purple |
| Cr3+ | Violet |
| Mn2+ | Pale pink |
| CrO42- | Orange |
| Cr2O72- | Yellow |
Where are transition metals found?
Certain transition metals like iron, copper, titanium, and mercury can be found in the earth’s crust, with iron being the most abundant. Copper can also be found on the surface of the earth and has been used for making useful tools since ancient times [11].
Uses and Application of Transition Metals
Transition metals have a wide variety of applications [12].
- Iron is used in steel which is a construction material in buildings, ships, vehicles, etc.
- Alloys of titanium are used in aircraft and in artificial knee and hip joints.
- Copper, being ductile and conducting, is used to make electrical wires and water pipes.
- Nickel and chromium are used in stainless steel, which is practically found in every kitchen item. Chromium, particularly, is responsible for the shiny color of stainless steel.
- Zinc is used as an anti-corrosion coating in galvanized steel.
- Cobalt is used in magnetic alloys and also in engines and jet turbines.
- Gold and silver are used in jewelry. Silver is also used in making dishes (silverware) and in many electronic applications.
- Platinum is used in jewelry, thermometers, catalytic converters, and rocket engines.
- Manganese and vanadium are added to steel to make it wear-resisting.
References
- Definition of transition metals – Britannica.com
- Location of transition metals in periodic table – Chemed.chem.purdue.edu
- List of transition metals – Coolperiodictable.com
- List of transition metals – Toppr.com
- Table of transition metals – Study.com
- Properties of transition metals – Chem.libretexts.org
- Chemical properties of transitional metals – Chem.libretexts.org
- Magnetic properties of transition metals – Courses.lumenlearning.com
- Color of transition metals – Chemistry.stackexchange.com
- Table of colors – Thoughtco.com
- Availability of transition metals – Scienceclarified.com
- Applications of transition metals – Schooledbyscience.com