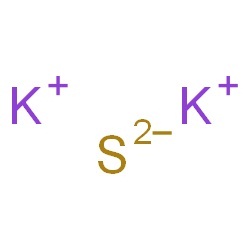Potassium Sulfide
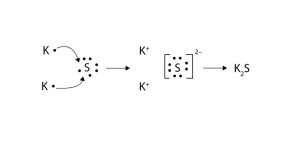
Potassium sulfide represented by the chemical formula K2S is a compound of potassium and sulfur that is moderately soluble in acids [1]. It is deliquescent and may spontaneously ignite in air. It is a reducing agent and an ionic compound [4].
Potassium Sulfide Identification |
|
| CAS Number | 1312-73-8 [1] |
| PubChem CID | 20072150 [3] |
| ChemSpider ID | 142491 [2] |
| EC Number | 215-197-0 [1] |
Composition and Synthesis
Potassium sulfide can be prepared by first treating potassium hydroxide to excess hydrogen sulfide to form potassium hydrosulfide (KHS). Further treatment of KHS with the same amount of potassium hydroxide generates potassium sulfide [9].
KOH + H2S = KHS + H2O
KHS + KOH = K2S + H2O
Properties and Characteristics of Potassium Sulfide
General Properties |
||
| Molar mass/molecular weight | 71.158 g/mol [3] | |
Physical Properties |
||
| Color/appearance | White to yellow powder [4] | |
| Melting point/freezing point | 840°C, 1544°F [1] | |
| Boiling point | 912°C, 1674°F [1] | |
| Density | 1.80 g cm-3 [1] | |
| State of matter at room temperature (normal phase) | Solid [1] | |
Chemical Properties |
||
| Solubility in water | N/A [1] | |
| pH | >7 (basic) [5] | |
Atomic Properties |
||
| Crystal structure | Tetrahedral [6] | |
Prominent Reactions of K2S
Potassium sulfide reacts with cobalt iii bromide to produce cobalt iii sulfide and potassium bromide [10].
3K2S + 2CoBr3 = Co2S3 + 6KBr
Potassium sulfide reacts with dilute hydrochloric acid to produce potassium chloride and hydrogen sulfide [11].
K2S + 2HCl = 2 KCl + H2S
It reacts with concentrated sulfuric acid to give potassium bisulfate, sulfur dioxide, sulfur and water [13]
K2S + 3H2SO4 = 2KHSO4 + SO2 + S + 2H20
The compound reacts with silver nitrate to form aqueous potassium nitrate and silver sulfide precipitate [12].
K2S + 2AgNO3 = 2KNO3 + Ag2S
Potassium Sulfide Uses
- In pyrotechnics [7].
- As a reagent in analytical chemistry [4].
- As a depilatory and medicine [4].
Is It Dangerous
It may cause a fire hazard, so precautions must be taken not to bring it in contact with air. In the form of powder or dust, it is explosive. Ingestion, inhalation and skin contact may result in severe injury and even death. It also harmfully affects the eyes and skin causing eye damage and skin burns. Hence all contact should be avoided. It emits toxic fumes if heated to decomposition [4]. It is of a corrosive nature and poisonous for the environment [8].
- References
- Potassium Sulfide – Americanelements.com
- Potassium Sulfide – Chemspider.com
- Potassium Sulfide – Pentachemicals.eu
- Potassium Sulfide – Chemicalbook.com
- Is K2S acidic or basic? – Quora.com
- Dipotassium Sulfide – Webelements.com
- What are the uses of potassium sulfide? – Quora.com
- Potassium Sulfide – Merckmillipore.com
- Preparation of potassium sulfide – Prepchem.com
- Solubility Rules and Net Ionic Equations Practice – Vincentianacademy.org
- Potassium sulfide react with hydrogen chloride – Chemiday.com
- Question #e42f1 – Socratic.org
- Potassium sulfide react with sulfuric acid – Chemiday.com