Iron
What is Iron
Iron (prounounced as EYE-ren) is a hard metal with a high commercial value, belonging to the family of transition metals. Represented by the chemical symbol Fe, it is chemically reactive with a tendency to corrode easily in air forming a reddish layer called rust when exposed to damp air [1, 2].
Where is Iron Found
It is the fourth most abundant metal in the earth’s crust, commonly associated with other mineral ores like hematite, taconite, and magnetite found in mining reserves of Ukraine, Brazil, Russia, Australia, and China. Its commercial production is carried out in a blast furnace by heating the ores with coke and limestone [1].
History
Origin of its Name: The name of the element comes from an Anglo-Saxon word ‘iron’.
Who Discovered it: Unknown
When, Where, and How was it Discovered
Around 3500 BC, the Egyptians were believed to be using iron objects. Hittites from Asia Minor were known to smelt the metal from its ores during 1500 BC. In 1722, René Antoine Ferchault de Réaumur, a French entomologist published a book, describing the significance of different iron alloys [1].
Identification |
|||
| Atomic number | 26 [1] | ||
| CAS number | 7439-89-6 [1] | ||
| Position in the periodic table [1] | Group | Period | Block |
| 8 | 4 | d | |
Classification, Properties and Characteristics of Iron
General Properties |
||
| Relative atomic mass | 55.845 [1] | |
| Atomic mass/weight | 55.845 atomic mass units [3] | |
| Molar mass/Molecular weight | 55.845 g/mole [6] | |
| Mass Number | 56 | |
Physical Properties |
||
| Color/physical appearance | Silver gray [1] | |
| Melting point/freezing point | 1538°C (2800°F) [1] | |
| Boiling point | 2861°C (5182°F) [1] | |
| Density | 7.87 g/cm3 [1] | |
| Standard/Natural state at room temperature (solid/liquid/gas) | Solid [1] | |
| Malleability | Yes | |
| Ductility | Yes | |
| Hardness | 4-5 Mohs [7] | |
| Specific heat capacity | 0.444 J g-1 oC [8] | |
| Thermal conductivity | 80.4 Wm-1K-1 [3] | |
Chemical Properties |
||
| Flammability | Not flammable [9] | |
| Oxidation state/Oxidation number | -2, -1, +1, +2, +3, +4, +5, +6 [1] | |
Atomic Data of Iron (Element 26)
| Electron configuration (noble gas configuration) | [Ar] 3d64s2[1] | ||||||
| Atomic structure [5] | |||||||
| – Number of Electrons | 26 | ||||||
| – Number of Neutrons | 30 | ||||||
| – Number of Protons | 26 | ||||||
| Radius of atom | |||||||
| – Atomic Radius | 2.04 Å [1] | ||||||
| – Covalent Radius | 1.24 Å [1] | ||||||
| Ionization energy [1]
(kJmol-1) |
1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| 762.466 | 1561.876 | 2957.469 | 5287.4 | 7236 | 9561.7 | 12058.74 | |
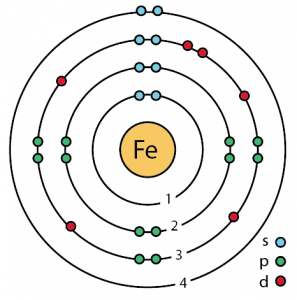
Iron Atomic Structure (Bohr Model)
What are the Common Uses of Iron
- Iron is used in the manufacture of different types of steel by alloying with other elements like carbon, nickel, chromium, and tungsten for making cutting equipment, bicycle chains, rifle barrels, transmission towers, bridge girders, and reinforced concrete [1].
- Stainless steel containing a high percentage of iron along with other metals has enormous strength and better function that’s useful in the making of surgical instruments, paper clips, cutlery, ball bearings, and jewelry [1, 2].
- Another form of Fe called wrought iron obtained by smelting is used to make carpenter tools, lifting hooks, chains, fence, and gates [10].
- Iron fillings are applicable in electromagnetism science experiments to assess the strength of magnets as well as in power metallurgy, artwork, fireworks, and sand blasting [11].
- Fe acts as an efficient catalyst is some industrial chemical processes such as Haber process and Fischer-Tropsch [1].
Does the Element Have Any Toxic Effects
Accidental ingestion of Fe has been associated with vomiting, diarrhea, and other gastrointestinal issues, a common type of metal poisoning. Prolonged accumulation in the body could result in respiratory problems and heart disorders [12]. Inhalation of the metal dust or fumes can cause a severe pulmonary reaction [13].
Interesting Facts
- Meteorites are believed to have high iron content [5].
- Iron was used as a magnetic metal by ancient navigators in the form of lodestones for making compasses [3].
- In an average human body, 4 grams of Fe is present, associated with hemoglobin that in turn helps in carrying oxygen to the lungs [1].
Iron Metal Price
The cost of pure iron may vary between $0.24 and $0.30 per pound.
- References
- http://www.rsc.org/periodic-table/element/26/iron
- https://education.jlab.org/itselemental/ele026.html
- https://www.chemicool.com/elements/iron.html
- https://www.radiochemistry.org/periodictable/elements/26.html
- https://www.thoughtco.com/iron-facts-606548
- Lbma.org.uk
- Lbma.org.uk
- http://www2.ucdsb.on.ca/tiss/stretton/database/specific_heat_capacity_table.html
- https://www.angelo.edu/faculty/kboudrea/demos/burning_iron/burning_iron.htm
- https://extrudesign.com/wrought-iron-properties-applications/
- http://www.iron-filing.com/
- https://www.clinicaladvisor.com/labmed/toxicity-associated-with-iron/article/614895/
- https://www.ncbi.nlm.nih.gov/pubmed/1592301









This is an in depth article on iron. It covered almost all aspects. Thanks for such an wonderful article