Boron
What is Boron
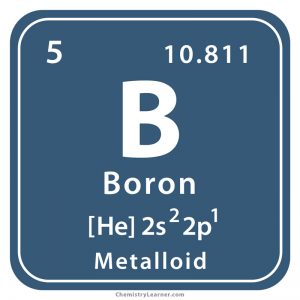
Boron (pronunciation BO-ron [2]), represented by the chemical symbol or chemical formula B [1], is hard and brittle in its crystalline form [22]. It has allotropes in the form of an amorphous powder and three major crystalline forms [34]. Naturally occurring B has two stable isotopes with mass numbers 10 and 11 [1, 2]. Besides that, it has 11 synthetic isotopes, some of which are radioactive, with mass numbers ranging from 7 to 17 that have known half-lives [3]. It doesn’t chemically react with air (oxygen) at normal temperature but does so at higher temperatures to form its oxide. It doesn’t normally react with water but reacts vigorously with halogens to form boron halides [11].
Where Is It Found
On earth, the metalloid can be found in volcanic spring waters as orthoboric acid and in the minerals colemanite and borax as borates. Large borax deposits are present in Turkey. However, the most important source is the mineral rasorite that has abundance in the Mojave Desert in California, USA [1]. It has a natural source in groundwater due to leaching from soils and rocks containing borosilicates and borates [33]. It has a variety of functions in almost all living things, is a trace element in humans and an essential micronutrient of plants [23, 29].
The human body should have a daily intake of 2 milligrams of B from his diet. Raisins, almonds, prunes and hazelnuts are boron-rich food [1, 8].
History
Origin of Its Name: Its name is derived from the Arabic word ‘buraq’, a term signifying the element [1, 2].
Who Discovered Boron and Where: French chemists, Louis-Josef Gay-Lussac and Louis-Jacques Thénard in Paris, France, and English chemist, Humphry Davy in London, UK, working independently [1, 2].
When Was It Discovered: Its discovery year is 1808 [1].
How Was It Discovered
For centuries Lake Yamdok Cho in Tibet was the only source of borax that was used by goldsmiths as a flux. In 1808, the French and English chemists mentioned above extracted the element independently by heating borax with potassium. However, they could not produce pure B which is very difficult to obtain. French chemist, Henri Moissan isolated a purer form in 1892. Eventually, in 1909, American chemist E. Weintraub successfully produced pure B in the USA by sparking boron chloride vapor and hydrogen. It had very different properties from the varieties of B previously reported [1, 39].
Boron Identification |
|||
| Atomic Number | 5 [1] | ||
| CAS Number | 7440-42-8 [1] | ||
| Position in the periodic table | Group | Period | Block |
| 13 [1] | 2 [1] | p [1] | |
Properties and Characteristics of Boron Element
General Properties |
||
| Atomic mass | 10.81 atomic mass units [1] | |
| Atomic weight/relative atomic mass | 10.81 [1] | |
| Mass number | 11 [6] | |
| Molar mass/molecular weight | 10.81 g/mol [7] | |
Physical Properties |
||
| Color/appearance | Black [1, 3] | |
| Luster | Yes, a small amount [20] | |
| Odor | None [16] | |
| Texture | Rough [20] | |
| Ductility | Yes [24] | |
| Melting point/freezing point | 2077°C, 3771°F [1] | |
| Boiling point | 4000°C, 7232°F [1] | |
| Density | 2.34 g cm-3 [1] | |
| Physical state of matter at room temperature (normal phase) | Solid [1] | |
| Hardness (Mohs scale) | 9.3 [21] | |
| Electrical conductivity | 0.0001 S/m [21] | |
| Thermal Conductivity | 27 W/(m K) [21] | |
Magnetic Properties |
||
| Magnetic ordering | Diamagnetic [21] | |
| Magnetic susceptibility | -9.41×10-11 m3/mol [21] | |
Chemical Properties |
||
| Oxidation state/Oxidation number | +1, +2, +3[2] | |
Atomic Data of Boron (Element Number 5)
| Valence electrons/valency | 3 [4] | ||||||
| Quantum numbers | |||||||
| – n | 2 [4] | ||||||
| – ℓ | 1 [4] | ||||||
| – m ℓ | -1 [4] | ||||||
| – m s | +½ [4] | ||||||
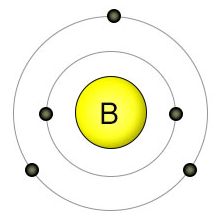
| Electron configuration (noble gas configuration) | [He] 2s22p1 [1] | ||||||
| Crystal structure | Rhombohedral [25] | ||||||
| Atomic structure | |||||||
| – Number of Electrons | 5 [3] | ||||||
| – Number of Neutrons | 6 [3] | ||||||
| – Number of Protons | 5 [3] | ||||||
| Energy levels [3] | |||||||
| – First Energy Level | 2 | ||||||
| – Second Energy Level | 3 | ||||||
| Radius of atom | |||||||
| – Atomic Radius | 1.92 Å [1] | ||||||
| – Covalent Radius | 0.84 Å [1] | ||||||
| Electronegativity (Pauling scale) | 2.04 [1] | ||||||
| Ionization energy
(kJmol-1) [1] |
1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| 800.637 | 2427.069 | 3659.751 | 25025.905 | 32826.802 | – | – | |
Boron Electron Configuration (Bohr Model)
Boron Uses
- Boron supplements are used as medicines to treat painful menstruation, osteoarthritis and rheumatoid arthritis, depression, building strong muscles and bones, increasing sex hormone testosterone levels. It benefits the body by increasing estrogen levels in post-menopausal women and healthy men. Estrogen helps in maintaining a sound mental and bone health [5]. The element also reduces skin aging, inflammation, brain fog, hair loss (it promotes hair growth), fungal infections and parasitic attacks of Candida [18, 19]. Boron deficiency can cause osteoporosis and psoriasis [26, 37].
- Being a good neutron absorber, boron 10 is used in the control rods of nuclear reactors, as a neutron detector and radiation shield [2].
- Its compound, sodium borate or borax is used in making eye drops, washing powders (laundry detergents), mild antiseptics, tile glazes, food and wood preservatives, bleach and other products that are used in everyday life [1].
- Boron compounds are used in organic fertilizers, insecticides, pesticides and agriculture [9].
- In boron neutron capture therapy for treating cancer and in neodymium magnets and fishing (fly) rods [13, 27].
- Borosilicate glass is an example of boron’s application in the glass and ceramic industry [14].
- Amorphous boron finds application as a rocket fuel igniter [1].
- Boron fibers or filaments are used in the construction of tapes with high tensile strength [15].
- It has importance in semiconductor doping [28]. For example, boron-doped diamond is an excellent material for electrodes [32].
- Nickel boron coating on firearms protects them from wear and tear [31].
- B is alloyed with steel, with the alloy named boron steel, to increase its hardness and prevent wear and tear [35].
- Burning with a green flame, it is used to make fireworks [36].
Is It Dangerous
Pure boron is not known to be harmful. However, too much of it can cause poisoning and is hazardous to the body’s metabolism [1]. It is considered safe during pregnancy in doses less than 20 mg per day, though it is best to consult with your doctor before taking it [30]. WHO recommends the boron level in drinking water should not exceed 2.4 mg/L [38].
Interesting Facts
- The largest borax mine in the world is the Rio Tinto Boron Mine in Boron, California, which produces nearly half of the world’s borates [10].
- Boron is the only metalloid of the boron family consisting of boron, aluminum, gallium, indium, and thallium [12].
- Boron buckyballs could further nanoparticle research and help in hydrogen storage [17].
Boron (B Element) Cost
The pure metal is priced at $1114 for every 100 gram and in bulk, it costs $500 for the same quantity [3].
- References
- http://www.rsc.org/periodic-table/element/5/boron
- https://education.jlab.org/itselemental/ele005.html
- https://www.chemicool.com/elements/boron.html
- http://chemistry-reference.com/q_elements.asp?Symbol=B&language=en
- https://www.webmd.com/vitamins/ai/ingredientmono-894/boron
- https://socratic.org/questions/what-is-the-mass-number-and-the-atomic-number-of-boron-11
- Pubchem.ncbi.nlm.nih.gov
- https://www.algaecal.com/algaecal-ingredients/boron/boron-sources/
- http://www.etimineusa.com/agriculture/
- http://www.riotinto.com/energyandminerals/boron-4638.aspx
- https://www.webelements.com/boron/chemistry.html
- https://www.learner.org/wp-content/interactive/periodic/groups9.html
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5296588/
- https://sciencestruck.com/boron-facts
- https://www.nature.com/articles/220781b0
- http://www.teacher.k12.de.us/~lettieri/pt/b/b.htm
- https://blogs.nature.com/news/2014/07/first-boron-buckyball-is-similar-to-carbon-original.html
- https://draxe.com/nutrition/boron-uses/
- https://www.organicfacts.net/health-benefits/minerals/boron.html
- https://prezi.com/pbsivz-gxypk/my-element-boron/
- http://periodictable.com/Elements/005/data.html
- https://www.thoughtco.com/boron-element-facts-606509
- Ods.od.nih.gov
- http://isbchem1.pbworks.com/w/page/9206122/Semi-Metals_E_Block
- https://www.webelements.com/boron/crystal_structure.html
- https://www.algaecal.com/algaecal-ingredients/boron/boron-benefits/
- https://www.intemag.com/neodymium-iron-boron
- https://www.halbleiter.org/en/fundamentals/doping/
- https://www.savvy-team.com/hww/boron-a-trace-element-essential-for-good-health/
- https://www.webmd.com/vitamins/ai/ingredientmono-894/boron
- http://rangehot.com/advantages-nickel-boronnitro-met-bcg-barrel-ar-rifle/
- https://www.egr.msu.edu/fraunhofer-ccd/sites/default/files/content/BDD_Corp.pdf
- Cdn.who.int
- http://www.chemistryexplained.com/A-Ar/Allotropes.html
- http://ispatguru.com/boron-in-steels/
- https://www.ducksters.com/science/chemistry/boron.php
- https://www.inspire.com/groups/talk-psoriasis/discussion/boron-vitamin-d-and-psoriasis/
- https://myhealth.alberta.ca/alberta/pages/Is-there-boron-in-my-drinking-water.aspx
- https://www.livescience.com/28674-boron.html