Praseodymium
What is Praseodymium
Praseodymium (pronunciation pra-see-oh-DIM-ee-em [2]), represented by the chemical symbol Pr [1], is a soft transition element [8] belonging to the family of rare earth metals [3, 4]. Naturally occurring Pr has a single stable isotope with mass number 141 [1, 3]. Besides that, it has 38 synthetic isotopes with mass numbers ranging from 121 to 159 that have been found to be radioactive [5, 6]. It is reactive with air, water, halogens, acids and bases [17].
Where Is It Found
The metal combined with other lanthanide elements can be found in many minerals, the principal ones being bastnaesite and monazite. It is extracted from them by ion exchange and solvent extraction [1]. It is mined in USA, China, Brazil, Australia, India and Sri Lanka mainly. Pr reserves are estimated to be 2 million tones approximately. World production is around 2500 million tonnes per year [18].
History
Origin of Its Name: Its name is derived from the Greek words ‘prasios didymos’ meaning green twin [1]. It is because of the green color its salts and close association with didymium [3, 4].
Who Discovered It: Austrian chemist, Carl Auer von Welsbach [1].
When Was Praseodymium Discovered: Its discovery year is 1885 [1].
How Was It Discovered
In 1841 Swedish chemist Carl Gustav Mosander extracted didymium and lanthanum from cerium. Didymium was considered to be an element for more than 40 years though it was actually a mixture of lanthanoid elements. Some chemists raised suspicions about its actual nature that were further confirmed by the findings of the Czech chemist Bohuslav Brauner in 1882. He revealed that its atomic spectrum did not resemble that of a pure metal. Welsbach took up the challenge and successfully split didymium into praseodymium and neodymium (in their oxide forms) in Vienna in June 1885. A pure sample of Pr metal was first produced in 1931 [1, 3, 4].
Praseodymium Identification |
|||
| Atomic Number | 59 [1] | ||
| CAS Number | 7440-10-0 [1] | ||
| Position in the periodic table | Group | Period | Block |
| Lanthanides [1] | 6 [1] | f [1] | |
Properties and Characteristics of Praseodymium
General Properties |
||
| Atomic mass | 140.908 atomic mass units [1] | |
| Atomic weight | 140.908 [1] | |
Physical Properties |
||
| Color/appearance | Silvery-white [1] | |
| Luster | Metallic [14] | |
| Odor | Odorless [15] | |
| Malleability | Yes [22] | |
| Ductility | Yes [22] | |
| Melting point/freezing point | 931°C, 1708°F [1] | |
| Boiling point | 3520°C, 6368°F [1] | |
| Density | 6.77 g cm-3 [1] | |
| State of matter at room temperature (normal phase) | Solid [1] | |
| Hardness (Mohs scale) | 1.41 [13] | |
| Electrical conductivity | 14.706 1/mohm-cm [7] | |
| Thermal Conductivity | 12.5 J/m-sec-deg [7] | |
Magnetic Properties |
||
| Magnetic ordering | Paramagnetic [13] | |
| Magnetic susceptibility | 5.9604×10-8 m3/mol [13] | |
Chemical Properties |
||
| Flammability | Yes [20] | |
| Oxidation state/Oxidation number | +2, +3, +4 [2] | |
Atomic Data of Praseodymium (Element 59)
| Valence electrons | 4 [13] | ||||||
| Quantum numbers | |||||||
| – n | 4 [16] | ||||||
| – ℓ | 3 [16] | ||||||
| – m ℓ | -1 [16] | ||||||
| – m s | +½ [16] | ||||||
| Electron configuration (noble gas configuration) | [Xe] 4f36s2 [1] | ||||||
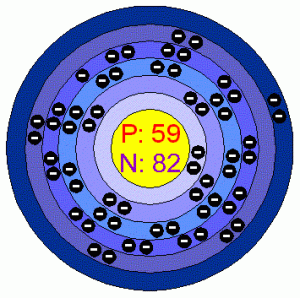
| Atomic structure | |||||||
| – Number of Electrons | 59 [3] | ||||||
| – Number of Neutrons | 82 [3] | ||||||
| – Number of Protons | 59 [3] | ||||||
| Energy levels [3] | |||||||
| – First Energy Level | 2 | ||||||
| – Second Energy Level | 8 | ||||||
| – Third Energy Level | 18 | ||||||
| – Fourth Energy Level | 21 | ||||||
| – Fifth Energy Level | 8 | ||||||
| – Sixth Energy Level | 2 | ||||||
| Radius of atom | |||||||
| – Atomic Radius | 2.40 Å [1] | ||||||
| – Covalent Radius | 1.90 Å [1] | ||||||
| Ionic Radius | 1.13 Å [19] | ||||||
| Ionic charge | +3 [10] | ||||||
| Electronegativity (Pauling scale) | 1.13 [1] | ||||||
| Ionization energy
(kJmol-1) [1] |
1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| 528.064 | 1017.92 | 2086.399 | 3761 | 5550.8 | – | – | |
Praseodymium Electron Configuration (Bohr Model)
Praseodymium Uses
- It is used in various alloys. The strong alloy that it forms with magnesium finds application in aircraft engines. It constitutes about 5% of Mischmetal that is used to make flints for cigarette lighters. It is also included in alloys that make permanent magnets [1].
- It finds application in carbon arc electrodes, along with other lanthanides, for studio projection and lighting [1].
- Its salts give an intense and unusually clean yellow color to glazes, glasses and enamel [1].
- Its oxide is a component of didymium glass that is used by glassmakers and welders to protect themselves from infrared (heat) radiation and filter out yellow light [1].
- It is doped into fiber optic cables for use as a signal amplifier [2]. It is also a doping agent in fiber lasers and garnets [11, 12].
- It finds application in nickel metal hydride (NiMH) rechargeable batteries of hybrid automobiles [3].
Is It Dangerous
Pr has low to moderate toxicity [1, 3]. The metal dust can cause a fire and explosion hazard [21].
Interesting Facts
- High pressure (up to 40 GPa) X-ray diffraction studies on the metal revealed the participation of its f-electrons in bonding that is in close resemblance to cerium and americium [9].
Praseodymium (Pr Element) Cost
The pure metal is priced at $470 for every 100 gram [3].
- References
- http://www.rsc.org/periodic-table/element/59/praseodymium
- https://education.jlab.org/itselemental/ele059.html
- https://www.chemicool.com/elements/praseodymium.html
- https://www.livescience.com/37655-praseodymium.html
- https://www.britannica.com/science/praseodymium
- http://www.softschools.com/facts/periodic_table/praseodymium_facts/364/
- https://www.lookchem.com/Periodic-Table/Praseodymium/
- https://quizlet.com/28814990/the-periodic-table-and-periodic-law-chapter-6-flash-cards/
- https://www.researchgate.net/publication/230941968_F_bonding_in_praseodymium_under_high_pressure
- http://www.knowledgedoor.com/2/elements_handbook/praseodymium.html
- Americanelements.com
- https://journals.aps.org/prb/abstract/10.1103/PhysRevB.34.7918
- http://periodictable.com/Elements/059/data.html
- http://metals.comparenature.com/en/what-is-praseodymium/model-81-0
- Pubchem.ncbi.nlm.nih.gov
- http://chemistry-reference.com/q_elements.asp?Symbol=Pr&language=en
- https://www.webelements.com/praseodymium/chemistry.html
- https://www.lenntech.com/periodic/elements/pr.htm
- https://chemglobe.org/ptoe/_/59.php
- http://www.elementsdatabase.com/Praseodymium-Pr-59-element/
- https://www.webelements.com/praseodymium/biology.html
- http://www.chemistryexplained.com/elements/P-T/Praseodymium.html