Cyclic Voltammetry
Cyclic voltammetry is a powerful electrochemical technique used to study the redox reactions of compounds in solution. Researchers can obtain valuable information about the electron transfer processes at the electrode surface by applying a potential sweep to an electrochemical cell and measuring the resulting current. [1-4]
Cyclic voltammetry involves cycling the potential of an electrode between two limits at a specific scan rate while recording the current response. It allows researchers to investigate the oxidation and reduction behavior of analytes, determine their redox potentials, and study reaction kinetics.
Equations
Cyclic voltammetry (CV) analysis relies on fundamental equations to interpret electrochemical data accurately. Central to understanding redox processes in CV is the Nernst equation, a fundamental relation that elucidates the relationship between the redox potential, the concentration of species, and temperature, thereby facilitating the interpretation of experimental data in cyclic voltammetry. [3]
E = E0 – (RT/zF) ln Q
Where:
E is the reduction potential
- E0 is the standard potential
- R is the universal gas constant
- T is the temperature in kelvin
- z is the ion charge (moles of electrons)
- F is the Faraday constant
- Q is the reaction quotient
When scientists and engineers use cyclic voltammetry along with the Nernst equation, they can better understand electrochemical systems. It helps them study basic rules and create new technologies related to electrochemistry. It is especially important for figuring out energy storage and medical sensors.
While cyclic voltammetry is the experimental technique used to collect data, cyclic voltammogram is the visual representation of that data. Together, they provide insights into a system’s electrochemical behavior.
Cyclic Voltammogram
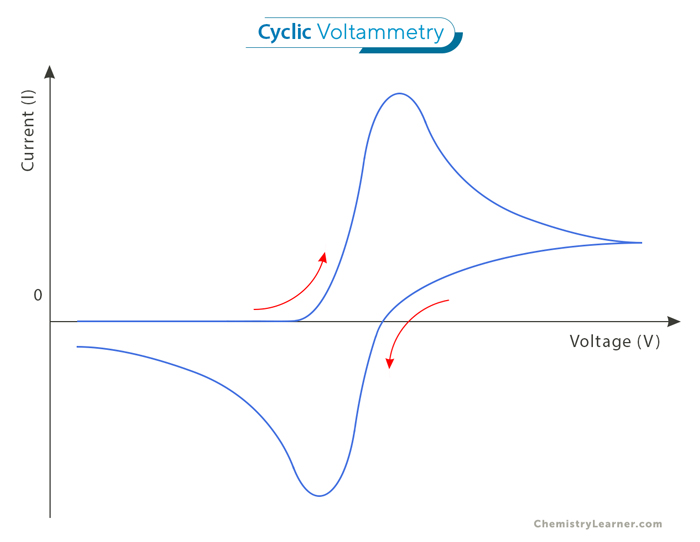
The cyclic voltammogram illustrates potential along the x-axis and current along the y-axis. However, there are two conventions for plotting the data: the American and IUPAC conventions. In the American convention, positive potential is depicted from right to left, with reduction currents represented as positive (while oxidation currents are negative). Conversely, the IUPAC convention displays positive potentials from left to right, with oxidation currents as positive. The IUPAC convention is predominantly used because of the prevalence of digital data collection and computer preferences for positive numbers. [3]
The figure above, depicting a representative cyclic voltammogram, shows the current-potential profile of a cyclic voltammetry experiment. As the potential is scanned towards more positive values, a point is reached where the electroactive species in the solution begin to undergo reduction. It increases current until a peak is reached, indicating that the current is then limited by the diffusion of additional reactant to the surface. Subsequently, as the potential becomes more positive, the diffusion layer near the electrode expands, resulting in a slower diffusion of additional reactant and a subsequent decrease in current. Upon reaching the potential limit, the current decreases until the potential reaches a point where oxidation of the reduced species near the surface occurs. This reversal leads to an increase in current in the opposite direction, culminating in a peak before decreasing until the initial potential is reached.
Key Parameters from Voltammogram
The voltammogram yields information crucial for comprehending electrochemical (redox) reactions. [3]
1. Redox Potential (E°): Cyclic voltammetry facilitates the assessment of the redox potential of an electroactive species. In reversible systems, the halfway potential between the peaks signifies equilibrium between oxidized and reduced species, offering a straightforward estimate of E0, the standard potential.
2. Electrochemical Reversibility: The cyclic voltammogram’s shape indicates the reversibility of the redox process—symmetrical peaks denote a reversible reaction, while asymmetrical peaks signify irreversibility.
3. Diffusion Coefficient (D): Through cyclic voltammetry, the diffusion coefficient of electroactive species can be inferred by conducting experiments at various scan rates. The Randles-Sevcik equation elucidates the relationship between peak current and scan rate, providing insights into species behavior—freely diffusing or adsorbed onto electrode surfaces.
4. Electroactive Species Concentration: The current peaks’ magnitude in cyclic voltammetry correlates directly with the concentration of participating electroactive species, facilitating concentration estimation.
5. Kinetic Parameters and Reaction Mechanism: Analysis of peak size, shape, and position enables the deduction of electron transfer rates, reaction kinetics, and the number of electrons involved in the redox process, shedding light on the reaction pathway.
6. Electrode Surface Area: Cyclic voltammetry may estimate electrode surface area, particularly for redox-active species with known diffusion coefficients.
7. Electrochemical Reaction Mechanisms: The cyclic voltammogram unveils electron transfer numbers and reaction pathways, offering crucial insights into reaction mechanisms.
Applications
This versatile technique finds applications across diverse fields owing to its simplicity and affordability. [3]
1. Electrochemical Sensors: Cyclic voltammetry detects analytes based on their redox activity, supporting glucose monitoring and pollutant detection applications.
2. Battery Research: Researchers utilize cyclic voltammetry to study battery materials, optimize performance, and understand degradation mechanisms.
3. Electroplating and Electrodeposition: For thin film deposition control, cyclic voltammetry optimizes parameters in electroplating and electrodeposition processes.
4. Medicine and Pharmaceuticals: In pharmaceutical research, cyclic voltammetry analyzes drug redox properties, aiding stability and interaction studies.
5. Fuel Cells: Cyclic voltammetry aids fuel cell development by evaluating catalysts and understanding electrochemical processes.
6. Electrochemical Capacitors (Supercapacitors): Cyclic voltammetry characterizes material behavior during charging and discharging for supercapacitor optimization.