Amide
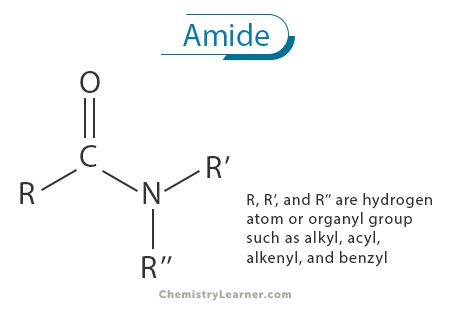
An amide is a fundamental class of organic compounds characterized by their distinct chemical structure, consisting of a carbonyl group (C=O) bonded to a nitrogen atom (N). This carbonyl-nitrogen linkage is a defining feature of amides and imparts unique properties and reactivity to these compounds [1-4].
Structure
Amides are often considered derivatives of carboxylic acids, where the hydroxyl (-OH) group of the carboxylic acid is replaced by an amino (-NH2) group. This substitution results in the formation of the amide linkage, represented as -CONH2. The fundamental structure of an amide consists of a carbonyl group (C=O) bonded to a nitrogen atom (N) with a single bond, and the nitrogen atom is also bonded to two additional substituents, typically hydrogen atoms (H). Thus, the central carbon atom in the carbonyl group is bonded to three distinct atoms or groups: a double-bonded oxygen atom, a nitrogen atom, and a substituent group (R) that can vary in size and complexity [1-5].
Classification
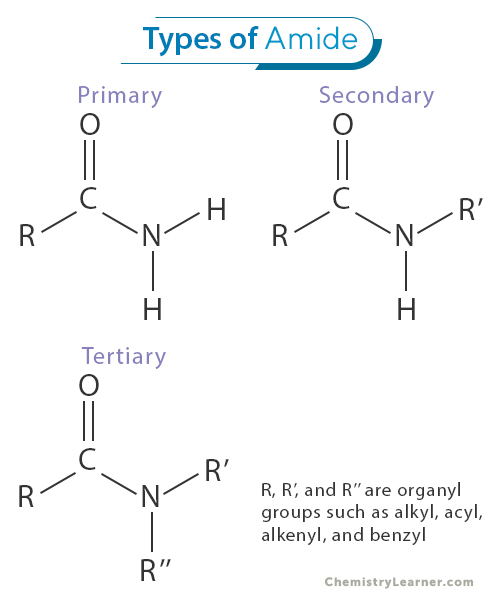
Amides can be classified into three main categories based on the number of alkyl or aryl groups attached to the nitrogen atom: primary, secondary, and tertiary amides [1-3].
Primary Amides
Primary amides are amides in which the nitrogen atom is bonded to one hydrogen atom and two carbon atoms. The general formula for primary amides is RCONH2, where R represents an alkyl or aryl group. Primary amides are often considered the simplest and most common type of amides. They find numerous applications in organic synthesis and the pharmaceutical industry due to their versatility in forming various compounds and functional groups.
Secondary Amides
Secondary amides have two alkyl or aryl groups and one hydrogen atom bonded to the nitrogen atom. Their general formula is RCONHR’, where R and R’ represent different alkyl or aryl groups. Secondary amides are intermediates in various chemical reactions and are often used to synthesize complex organic molecules. Their distinct structure allows them to participate in amide bond formation, which is crucial in peptide and protein synthesis.
Tertiary Amides
Tertiary amides are amides in which the nitrogen atom is bonded to three alkyl or aryl groups, with no hydrogen atoms directly attached to nitrogen. Their general formula is RCONR’R”, where R, R’, and R” represent different alkyl or aryl groups. Tertiary amides are relatively stable and less reactive than primary and secondary amides. They are commonly found in natural products and some pharmaceutical compounds.
Nomenclature
Naming amides using IUPAC rules generally involves identifying the parent carboxylic acid and the amine component from which the amide is derived. Here are the key steps [1,2]:
- Select the longest carbon chain containing the carbonyl group (C=O). The name of this carboxylic acid serves as the base name.
- Replace the “-oic acid” ending of the carboxylic acid name with “-amide.” This change indicates the presence of an amide functional group.
- Specify the substituents on the nitrogen atom of the amide group using prefixes like N-substituted alkyl or aryl groups.
- Number the carbon atoms in the parent chain to indicate the position of any substituents on the chain.
- List the substituents, their positions, and the base name, separated by hyphens. Arrange them alphabetically, ignoring any numerical prefixes like di- or tri-. Use numerical locants to indicate the positions of substituents.
Here are some examples:
- Acetamide is derived from acetic acid and given by the formula H3CCONH2.
- Benzamide is derived from benzoic acid with the formula C6H5CONH2.
- N-methylacetamide is an example of an amide with a common name that specifies a substituent on the nitrogen atom. It is derived from acetamide by replacing one of its hydrogen atoms with a methyl group. Its formula is H3CCONHCH3.
Synthesis
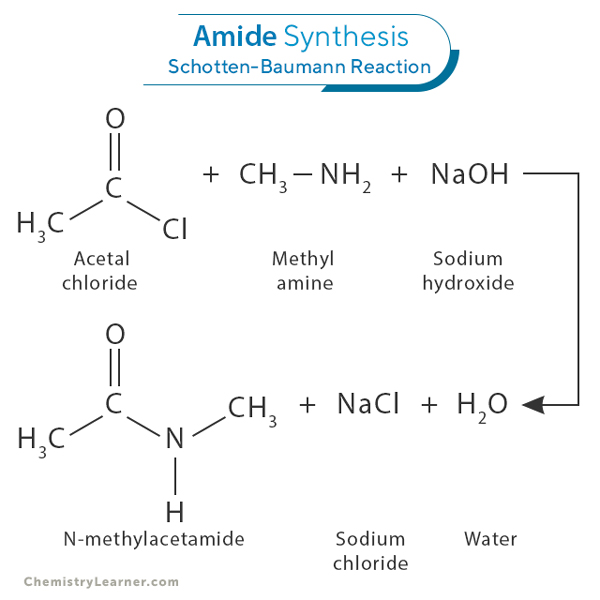
Amides can be synthesized through acylation, a standard method involving the reaction between an acyl chloride (RCOCl) and an amine (RNH2) in the presence of a base such as sodium hydroxide (NaOH). This process forms an amide bond, releasing water as a byproduct. This amide formation is known as the Schotten-Baumann reaction [1-3].
For example, acetal chloride (CH3COCl) reacts with methylamine (CH3NH2) in the presence of sodium hydroxide (NaOH) to produce N-methylacetamide (CH3CONHCH3). The reaction proceeds as follows:
CH3COCl + CH3NH2 + NaOH → CH3CONHCH3 + NaCl + H2O
Amide Hydrolysis
Amides exhibit intriguing chemical properties due to the carbonyl group’s presence within their structure. This carbonyl group is polarized, with the carbon atom being partially positively charged and the oxygen atom partially negatively charged. Consequently, amides can undergo various reactions, including hydrolysis. The reactivity of amides depends on the nature of the substituents attached to the nitrogen atom and the reaction conditions, making them valuable intermediates in organic synthesis [1,2].
One of the fundamental chemical properties of amides is their susceptibility to hydrolysis, a reaction in which water molecules break the amide bond. This hydrolysis can occur under both acidic and alkaline conditions. In acidic hydrolysis, the amide bond is cleaved to yield a carboxylic acid and an ammonium or ammine salt.
RCONH2 + H2O + H+ X– + heat → RCOOH + NH4+ X–
RCONHR’ + H2O + H+ X– + heat → RCOOH + R’NH3+ X–
In contrast, alkaline hydrolysis results in the formation of a carboxylic acid and ammonia or amine.
RCONH2 + H2O + heat → RCOOH + NH3
RCONHR’ + H2O + heat → RCOOH + R’NH2
Where X is a halide.