Amines
Amines are a fascinating class of organic compounds that play a crucial role in various biological, industrial, and medicinal processes. Their unique structure and versatile properties make them a subject of extensive research and application. [1-4]
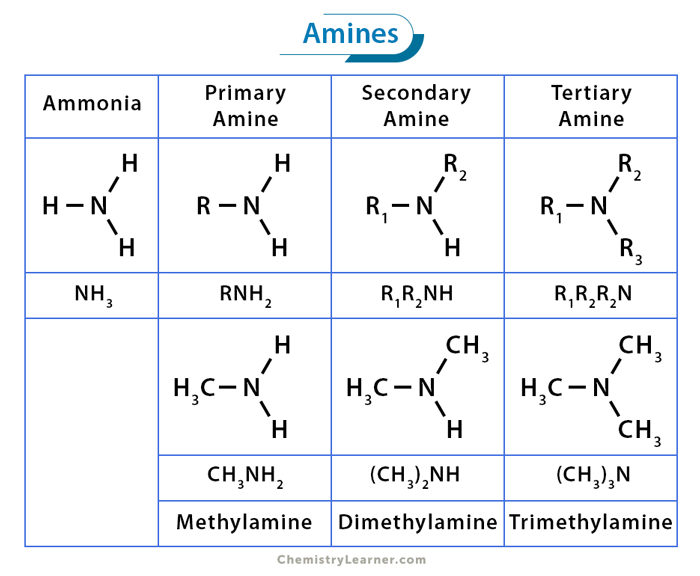
Amines are derived from ammonia (NH3), in which alkyl or aryl groups replace one or more hydrogen atoms. They are characterized by the presence of a nitrogen atom bonded to carbon atoms and hydrogen atoms. The general formula of an amine is RNH2, where R represents the alkyl or aryl group.
Structure and Types
The structure of amines revolves around the central nitrogen atom bonded to various substituents. Depending on the number of hydrogen atoms replaced by alkyl or aryl groups, amines can be categorized into three main types: [1-4]
1. Primary or 1° Amines
They have one hydrogen atom replaced by an alkyl or aryl group. The general structure is RNH₂. Examples include n-butylamine (CH3(CH2)3NH2) and aniline (C6H5NH2).
2. Secondary or 2° Amines
Two hydrogen atoms are substituted by alkyl or aryl groups. The general structure is RR’NH. Examples includes ethylmethylamine (C2H5(NH)CH3) and diphenyl amine (C6H5(NH)C6H5).
3. Tertiary or 3° Amines
They feature three alkyl or aryl groups bonded to the nitrogen atom. The general structure is RR’R”N. Examples include trimethylamine ((CH3)3N) and dimethylbenzylamine ((CH3)2NC6H5CH2).
A fourth subcategory also exists and is determined by the connectivity of the substituents attached to the nitrogen:
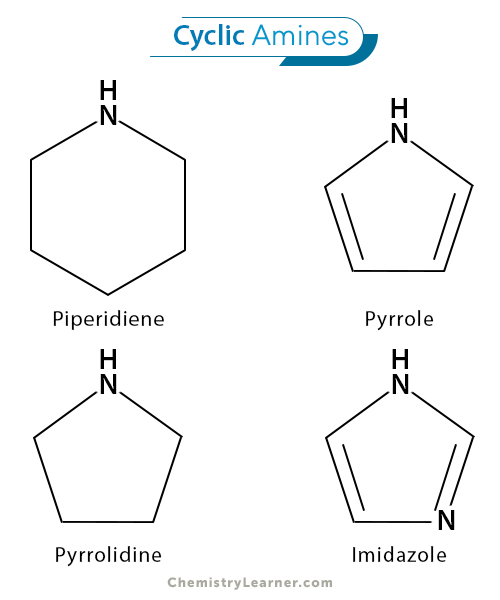
4. Cyclic Amines
They are either secondary or tertiary amines. They are part of a cyclic structure and are crucial components of many biologically active molecules. Pyrrole (C4H5N) and pyridine (C4H5N) are common examples.
Nomenclature
The systematic names of primary amines are derived from the name of the parent alkane by adding the prefix “-amino” or suffix “-amine” and a number specifying the carbon that carries the amino group. For example, 5-amino-2-hexane (CH3(CH2)3CHNH2CH3). [1-6]
Properties
Amines are characterized by their unique nitrogen-containing structure and exhibit various properties that distinguish them from other classes of organic compounds. [1,3]
1. Odor
One of the most distinctive properties of amines is their pungent odor. This odor is particularly pronounced in lower molecular weight amines, frequently described as having a fishy or ammonia-like smell. However, as the size and complexity of the amine molecules increase, their odors tend to be less noticeable.
2. Solubility
The solubility of amines in water varies depending on the size of the molecule and the presence of hydrogen bonding. Small primary amines, such as methylamine and ethylamine, are typically soluble in water due to the ability of their amino groups to form hydrogen bonds with water molecules. However, solubility decreases as the carbon chain length increases or when aromatic rings are present.
3. Boiling and Melting Points
Amines generally have lower boiling and melting points than compounds of similar molecular weight lacking the nitrogen atom. This physical characteristic is attributed to the relatively weak intermolecular forces in amines. Primary and secondary amines can form hydrogen bonds between their amino groups, leading to higher boiling points than tertiary amines.
4. Basicity
Amines are weak bases due to the lone pair of electrons on the nitrogen atom. This lone pair can accept a proton (H⁺), forming a positively charged ammonium ion (RNH₃⁺). The basicity of amines depends on their structure and the availability of the lone pair. Primary and secondary amines are generally more basic than tertiary amines. Aromatic amines, despite their electron-withdrawing substituents, still display appreciable basicity. [5,6]
Synthesis
A wide array of synthetic methods exists to produce primary, secondary, and tertiary amines, each tailored to specific starting materials and desired outcomes. We will focus our attention on one method. [2,5]
Gabriel Synthesis
The Gabriel synthesis is a method for preparing primary amines from phthalimide. The phthalimide is first treated with an alkyl halide to form an N-alkyl phthalimide. This intermediate is then hydrolyzed under basic conditions, resulting in the formation of the desired primary amine. The Gabriel synthesis is beneficial for synthesizing primary amines with no alpha-hydrogens.
Reactivity and Chemical Reactions
Amines exhibit a wide range of reactivity due to a lone pair of electrons on the nitrogen atom. This lone pair makes amines highly nucleophilic, allowing them to engage in various chemical reactions. [2,3,5]
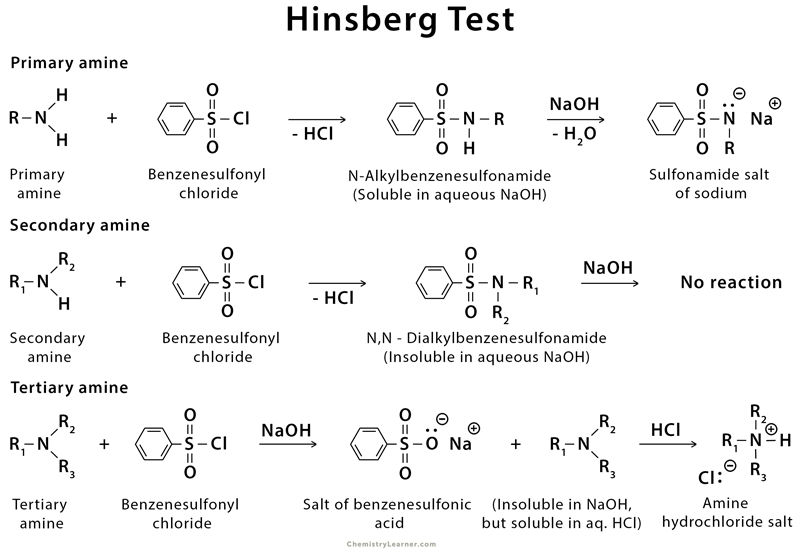
1. Hinsberg Test
The Hinsberg test is a chemical reaction that can distinguish between primary, secondary, and tertiary amines. The amine is shaken well with Hinsberg reagent, an aqueous solution of sodium hydroxide and benzenesulfonyl chloride, in the presence of aqueous alkali. The amine acts as a nucleophile and attacks the electrophilic benzenesulfonyl chloride, leading to the displacement of the chloride and the generation of the N-alkyl benzenesulfonamide. When the primary amine forms a sulfonamide, this product is soluble in aqueous sodium hydroxide. On the contrary, secondary and tertiary amine sulfonamide precipitate from the alkali solution as a solid.
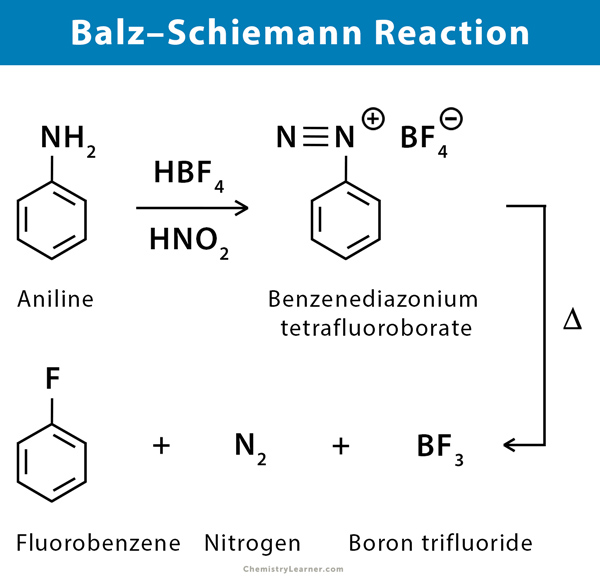
2. Reaction with Nitrous Acid (Diazotization) or Balz-Schiemann Reaction
Primary aromatic amines can undergo diazotization reactions in the presence of nitrous acid and fluoroboric acid. The process involves the conversion of arylamines to aryl fluorides via diazotization and subsequent thermal decomposition of the derived tetrafluoroborates.






It was quite helpful