Isomer
Molecules can vary by the way atoms are arranged in three-dimensional space. The same combination of atoms occupies space in different ways. Isomers are structures with the same molecular formula and chemical composition but different arrangements of atoms in space [1-4].
Types of Isomers
There are two main types of isomers: structural isomers and stereoisomers [1-8].
Structural Isomers
Structural isomers are also called constitutional isomers. They refer to the spatial arrangement of atoms and functional groups in the molecule. Structural isomers differ in how atoms and functional groups are attached to the molecular chain, resulting in different connectivities. Structural isomers are divided into several groups [1-8].
Skeletal Isomers
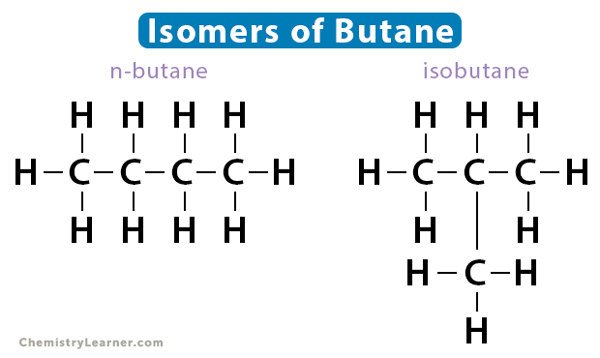
Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. The simplest hydrocarbons like methane, ethane, and propane do not have structural isomers. The smallest hydrocarbon that can display such isomerism is butane (C4H10), which has four carbon atoms. Its two isomers are i. n-butane (straight chain) and ii. isobutane (branched chain).
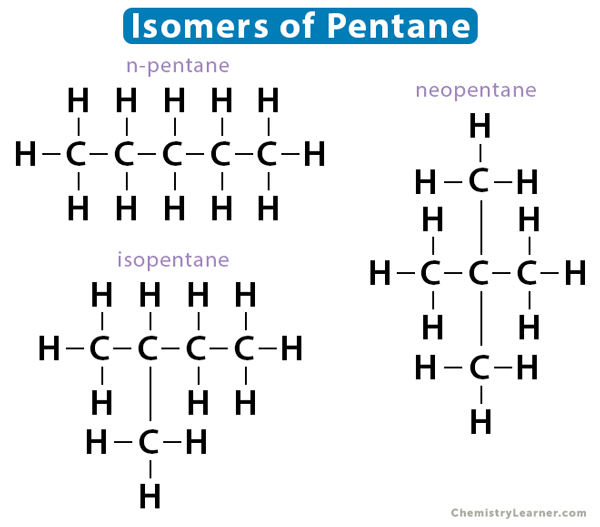
Likewise, pentane has three isomers – i. n-pentane, ii. isopentane, and iii. neopentane.
Positional Isomers
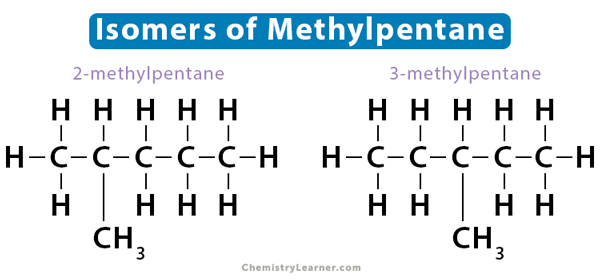
Positional isomers are also known as regioisomers. The functional group attaches to the hydrocarbon chain at different positions. As a result, the molecule exhibits different branching patterns. In the case of 2-methylpentane and 3-methylpentane, the position of the methyl functional group (-CH3) changes. The number on the compound represents the lowest-numbered carbon on which the functional group is located.
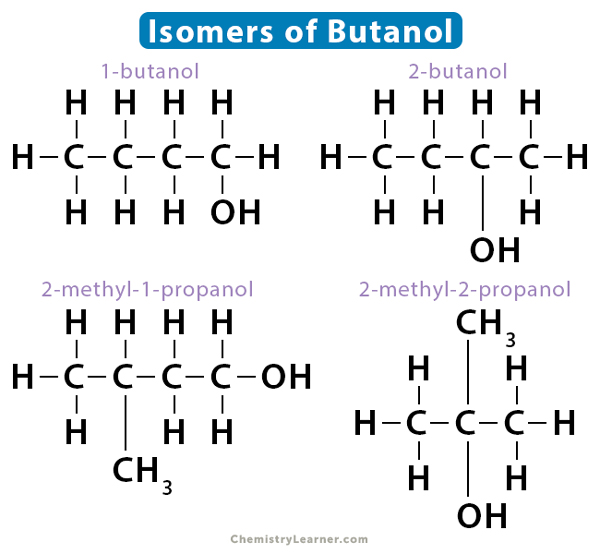
Similarly, butanol also displays positional isomerism, namely, 1-butanol and 2-butanol. However, it is not restricted to these two isomers only. It has two skeletal isomers – 2-methyl-1-propanol and 2-methyl-2-propanol.
Functional Isomers
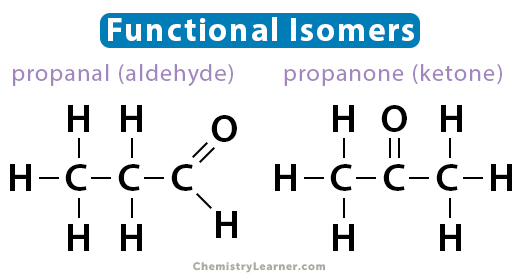
These structural isomers have different functional groups and significantly different physical and chemical properties. An example of a pair of functional isomers is propanal and propanone (acetone). Both have the molecular formula C3H6O. Propanal is an aldehyde, while propanone is a ketone. Another example pair is glucose and fructose.
Stereoisomers
Stereoisomers have the same connectivity of atoms but are arranged differently in space. The geometric positioning of atoms and functional groups are different. Molecules that are stereoisomers of each other represent the same structural isomer. There are several groups of stereoisomers [1-8].
Geometric Isomers
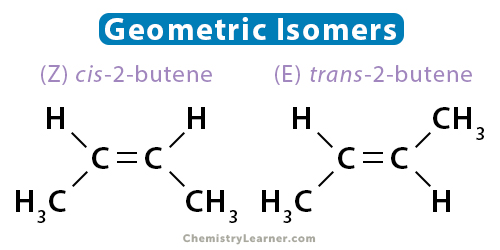
Geometric isomers have the same order of bonding but different arrangements of atoms in space. For geometric isomer to exist, the structure must be rigid and nonrotatable. Such properties are observed in alkenes and ringed structures. The two carbons in the double bond must have different groups attached to it. Geometric isomerism is also known as cis-trans isomerism or E-Z isomerism.
An example of geometric isomerism is 2-butene. There are two ways to draw the molecule, as shown below. The two isomers are known as cis-2-butene and trans-2-butene. In cis-2-butene, the two hydrogen atoms are on the same side of the molecule. In trans-2-butene, the two hydrogen atoms are on opposite sides.
The geometric isomers have different physical and chemical properties. Alkynes do not have geometric isomers since only one group is attached to the carbon atoms involved in the triple bond.
Optical Isomers
Those stereoisomers that are not geometric are called optical isomers. They differ in how functional groups are placed around one or more atoms in the main chain. They are called so because of their effect on plane-polarized light. There are two types of optical isomers.
1. Enantiomers
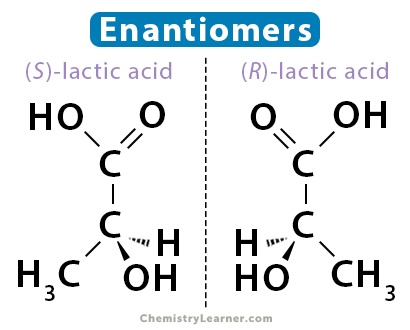
Enantiomers are mirror images of each other but are not superimposable. They are also called chiral and have chiral centers. A molecule is chiral if four unique groups are attached to a carbon atom. Unlike geometric isomers, enantiomers have the same physical and chemical properties.
Lactic acid consists of two enantiomers. One is (S)-lactic acid, and the other, its mirror image, is (R)-lactic acid. Also, lactic acid is chiral. Its central carbon atom is connected to four different groups – carboxylic acid (COOH), hydroxyl (OH), methyl (CH3), and hydrogen (H).
2. Diastereomers
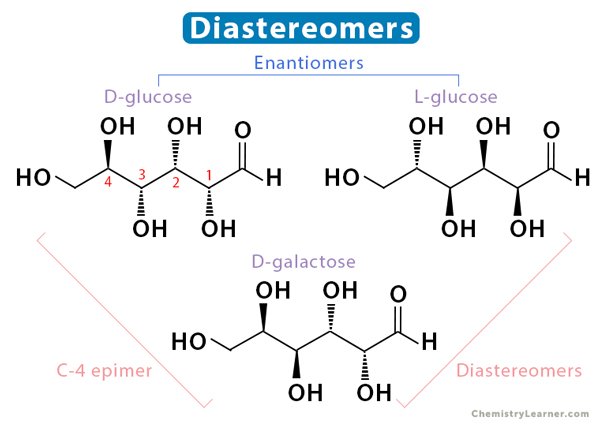
Diastereomers are non-mirror image isomers and are not superimposable. They may or may not have chiral centers. They have different arrangements of functional groups around some atoms and the same around others. A diastereomer is called an epimer when the change in arrangement is observed around one atom. Technically, cis– and trans-isomers are diastereomers. Unlike enantiomers, diastereomers have different physical properties.
In the image below, we observe that D-glucose and L-glucose are enantiomers. Also, D-galactose is a diastereomer of both D-glucose and L-glucose. However, it is a C-4 epimer of D-glucose.
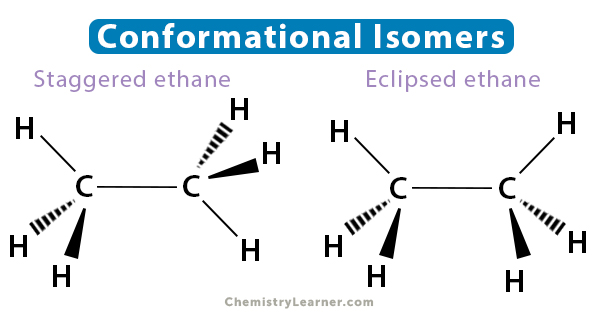
Conformational Isomers
Conformational isomers differ by their rotation about a sigma bond in the molecular chain. Only molecules with single bonds display conformational isomerism since they can rotate freely. For instance, ethane has two limiting structures. They are i. staggered ethane, where the carbon-hydrogen bonds are as far apart as possible, and ii. eclipsed ethane, where the bonds are as close as possible.