Polarity of Carbon Disulfide (CS2)
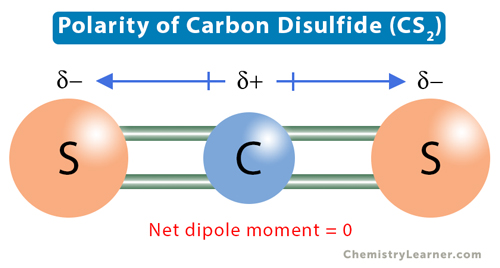
Carbon disulfide (CS2) has a carbon (C) atom surrounded by two sulfur (S) atoms. Carbon has four valence electrons, and sulfur has six. Each sulfur atom shares a double bond with the central carbon atom, completing the octets of all three atoms. Carbon does not have any lone electron pair. Therefore, the molecular structure of CS2 is linear.
The electronegativity of carbon is 2.55, and that of sulfur is 2.58. The electronegativity difference is 0.03. Therefore, the C-S bond is slightly polar. The directions of the two dipole moment vectors are equal and opposite. As a result, they cancel, making CS2 nonpolar.