Polarity of Nitrogen Bromide (NBr3)
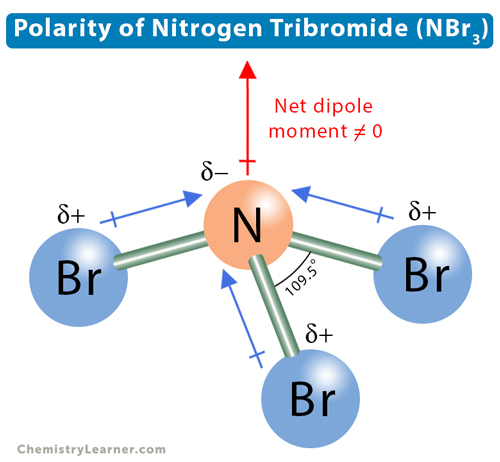
Nitrogen bromide (NBr3) has a central nitrogen (N) atom that is surrounded by three bromine (Br) atoms. N and Br chemically bond through single covalent bonds. Two factors affect the polarity of the molecule – electronegativity difference and molecular geometry.
The electronegativity difference between N and Br is low (0.08), and the N-Br single covalent bond can be considered slightly polar. There are three such covalent bonds in NBr, resulting in a trigonal pyramidal geometry with a bond angle of 109.5°. The lone electron pair on nitrogen repels the bonding pairs, making the molecule asymmetric. As a result, the entire molecule has positive and negative poles. Therefore, NBr is a polar molecule.